Raman spectra of functionalized carbon nanotubes (poster)
advertisement

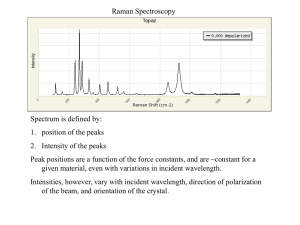
Raman spectra of functionalized carbon nanotubes G. Klupp, F. Borondics, R. Hackl*, K. Kamarás, E. Jakab**, S. Pekker Research Institute for Solid State Physics and Optics, Hungarian Academy of Sciences, Budapest, Hungary, e-mail: klupp@szfki.hu *Walther Meissner Institute, Bavarian Academy of Sciences and Humanities, Garching, Germany **Institute of Materials and Environmental Chemistry, Budapest, Hungary Resonant Raman scattering Tubes@Rice: Pulsed laser vaporization SWCNT + Ni/Co catalyst Refluxing with HNO3 SWCNT-COOH Heating to 800 ºC SWCNT Depth sampling Intensity (counts/s) 1300 S33+S44 468 nm Functionalization by modified Birch reduction [1]: Li + n NH3 Li+ + e- (NH3)n e- (NH3)n + C C- + nNH3 C- + BzBr BzC + BrC- + BuI BuC + IC- + MeI MeC + IC- + HX HC + X(HX = H2O, NH3, CH3OH) 531 nm S33 676 nm M11 140 1400 1500 1600 1700 1300 Intensity (counts/s) The samples Funding OTKA T 049338 Alexander von Humboldt Foundation Bz-, H-SWNT 531 nm laser 70 120 1400 1500 1600 1700 1500 1600 1700 Bu-, H-SWNT 531 nm laser 80 40 0 1300 1400 1500 1600 1700 1300 -1 1400 -1 Raman shift (cm ) Raman shift (cm ) The samples are inhomogeneous average spectra selected for comparison with TG-MS The degree of functionalization (R+H)/100C was determined from TG-MS. [2] A complete spectrum 900 1200 1500 3000 0.04 468 nm 531 nm 0.03 676 nm I (counts/s) 0.10 468 nm 676 nm 0.05 531 nm Gaussian 0.02 0.01 0.00 20 Bz 0 2 Bu Me 4 6 0 0 0 Lorentzian 40 0.15 0 Bu-,H-SWNT 531 nm laser ID/IG - ID /IG 60 600 0.05 ID/ID* - ID /ID* 300 Selectivity on tube type 0.00 -0.05 -0.10 8 Bz 0 (R+H)/100C 2 Bu Me 4 6 8 (R+H)/100C 0 300 600 900 1200 1500 3000 ID/IG and ID/ID* increase with the degree of functionalization, as the change of the electronic structure is only minor. The ratio depends on the wavelength of the exciting laser, as in Ref. 3. If we substract the value measured in the pristine sample (arising from the defects of the pristine nanotube) the change is similar for both metallic and semiconducting nanotubes. The reaction is not selective for tube type -1 Raman shift (cm ) No functional groups are visible and nanotubes are still in resonance. Electronic structure is not collapsed due to functionalization. The same degree of functionalization leads to smaller changes in the electronic structure in the case of apolar alkyl groups than in the case of polar substituted phenyl groups [3]. Selectivity on tube diameter I (arb. u.) 80 200 250 SWNT Me-,H-SWNT 468 nm 60 40 150 200 250 -1 Raman shift (cm ) I(small d RBM) / I(sum RBM) 150 Explanation of the selectivity Most of the functionalization reactions are primarily selective to metallic tubes [7], as these tubes have the nonzero DOS at the Fermi level [8]. Birch-type alkylation begins with doping by excess Li, which fills both S11, S22 and M11[9]. The selectivity for metallic tubes is masked 0.8 0.7 0.6 0.5 0.4 The charged nanotubes are dispersed in the liquid NH3 solution. The size of the cavity in the bundle does not play a role. 0.3 0.2 0 2 4 6 8 (R+H)/100C Carbanions having greater s-character are more stable. Smaller diameter tubes are more reactive According to the RBM spectrum the small diameter semiconducting nanotubes react more readily. This is in accordance with NIR[4, 5] and Raman[6] spectroscopic measurements on alkylated HiPCO tubes. In the case of 531 nm and 676 nm laser excitation the change was obscured by the error. References [1]: F. Borondics, E. Jakab, S. Pekker: Journal of Nanoscience and Nanotechnology 7, 1551 (2007) [2]: H. Kataura, Y. Kumazawa, Y. Maniwa, I. Umezu, S. Suzuki, Y. Ohtsuka, Y. Achiba: Synth. Metals 103, 2555 (1999) [3]: C. Fantini, M. L. Usrey, M. S. Strano: J. Phys. Chem. C 111, 17941 (2007) [4]: Á. Pekker, D. Wunderlich, K. Kamarás, A. Hirsch: Phys. Stat. Sol. B 245, 1954 (2008) [5]: K. Németh, F. Borondics, E. Jakab, Á. Pekker, K. Kamarás, S. Pekker: Poster #5 on SIWAN 2008 [6]: M. Müller, J. Maultzsch, D. Wunderlich, A. Hirsch, C. Thomsen: Phys. Stat. Sol. B 244, 4056 (2007) [7]: K. Kamarás, Á. Pekker: Handbook of Nanoscience and Technology, Editors: A. V. Narlikar, Y. Y. Fu, Oxford University Press, 2009 [8]: M. S. Strano: J. Am. Chem. Soc. 125, 16148 (2003) [9]: S. Kazaoui, N. Minami, R. Jacquemin, H. Kataura, Y. Achiba: Phys. Rev. B 69, 13339 (1999)