Reaction Rates and Equilibrium
advertisement
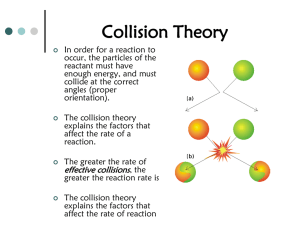
Reaction Rates and Equilibrium Chemistry Chapter 18 Rates of Reaction The rate of a chemical reaction is usually expressed as the change in the amount of a reactant per unit of time. Collision theory – particles collide & react if enough energy Activation energy – minimum energy required to react What factors affect reaction rates? The rate of a chemical reaction depends upon temp, concentration, particle size, and the use of a catalyst. A catalyst lowers the activation energy without taking part in the reaction. An inhibitor is a substance that interferes with a catalyst Reversible Reactions A reversible reaction is one in which the conversion of reactants to products and products to reactants occur simultaneously. When the forward & reverse reaction are equal the reaction has reached a state of balance; chemical equilibrium. At a chemical equilibrium, no net change occurs in the amounts of components of the system. Factors Effecting Equilibrium Equilibrium position shifts as a result of changing conditions. LeChaterler’s Principle – if stress is applied to a system in dynamic equilibrium, the system changes in a way that releases the stress. These stresses include: Changes in the concentration of reactants or products Changes in the temp Changes in the pressure LeChaterler’s Principle Suppose you have an equilibrium established between four substances A, B, C and D. Using Le Chatelier's Principle with a change of concentration What would happen if you changed the conditions by increasing the concentration of A? Using Le Chatelier's Principle with a change of pressure This only applies to reactions involving gases: What would happen if you changed the conditions by increasing the pressure? Using Le Chatelier's Principle with a change of temperature For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is released): Using Le Chatelier's Principle with a change of temperature (cont) What would happen if you changed the conditions by increasing the temperature? What would happen if you changed the conditions by increasing the temperature? Equilibrium Constant In a general reaction, a mol of reactant A and b mol of reactant B react to give c mol of product C and d mol of product D at equilibrium. The equilibrium constant (Keq) is the ratio of product concentrations to reactant concentrations at equilibrium, with each concentration raised to a power equal to the number of moles of that substance in the balanced chemical equation. A value of Keq greater than 1 means that products are favored over reactants; a value of Keq less than 1 means that reactants are favored over products. Solubility Product Constant The solubility product constant (Ksp), equals the product of the concentrations of the ions each raised to a power equal to the coefficient of the ion in the dissociation equation. The lower the numerical value, the lower the solubility of the compound. The Common Ion Effect If the product of the concentrations of two ions in the mixture is greater than the Ksp of the compound formed from the ions, a precipitate will form. A precipitate of barium sulfate forms as barium nitrate (Ba(NO3)2 and sodium sulfate (Na2SO4) solutions are mixed. Applying Concepts - What is the product of the concentrations of barium ion and sulfate ion after precipitation is complete? Free Energy & Spontaneous Reactions Excess energy released in a chemical reaction is referred as free energy as is thus available to do work. A spontaneous reaction is one that occurs naturally and favors the formation of substantial amounts of products at the specified conditions; also releases free energy. A nonspontaneous reaction is one that does not favor the formation of products at the specified conditions. Entropy Entropy is a measure of disorder in a system. The law of disorder states that the natural tendency of a system is to move in the direction of maximum disorder or randomness. An increase in entropy favors the spontaneous chemical reaction; decrease favors the nonspontaneous reaction. Applying Concepts - Which has the greater entropy, an assembled jigsaw puzzle, or the pieces in the box? Enthalpy, Entropy, & Free Energy The size & direction of enthalpy changes & entropy changes together determine whether a reaction is spontaneous; that is, whether it favors products and releases free energy. Gibbs Free-Energy All spontaneous reactions release some energy (“Gibbs” Free Energy) that becomes avalible to do work. Gibbs Free Energy change is the max. amount of energy that can be used to do work.. A change in Gibbs free energy is related to the change in entropy (ΔS) and the change in enthalpy (ΔH) of the system by the free-energy equation. ΔG = ΔH − TΔS (The temperature (T) is in kelvins.) The numerical value of ΔG is negative in spontaneous processes because the system loses free energy. Rate Laws A→B The rate at which A forms B can be expressed as the change in A (ΔA) with time, where concentration A1 is the initial concentration of A at time t1 and concentration A2 is the concentration of A at a later time, t2. Rate Laws (cont) A rate law is an expression for the rate of a reaction in terms of the concentration of reactants. The specific rate constant (k) for a reaction is a proportionality constant relating the concentration of reactants to the rate of the reaction. The value of the specific rate constant, k, is large if the products from quickly; the value is small if the products form slowly. Reaction Mechanisms Graphs (a reaction progress curve) of the progress of a reaction show peaks that correspond to the energies of activated complexes and valleys that correspond to the energies of intermediates & products.