Antimycobacterium
advertisement

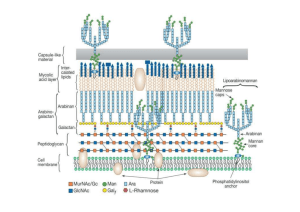
• Antimycobacterial Agents Mycobacteria: Mycobacterium tuberculosis => Tuberculosis Mycobacterium leprae => Leprosy (Hansen’s disease) • Slow-growing and difficult to stain (acid-fast bacilli). • Abnormally high lipid content (mycolic acid) of the cell envelope. • Treatment of mycobacterial infection complicated by: – Intracellular location of mycobacteria - phagocytes – Drug resistance – Chronic nature of these diseases • In tuberculosis, the bacilli reach the alveoli, ingested by pulmonary macrophages, fibroblasts then enclose the infection site leading to formation of granulomas or tubercles, hence the name Tubercle Bacillus (T.B). Diagram of cell wall • Leprosy primarily affects the skin as chronic granulomatous infection. • Tuberculosis primarily affects the lung (pulmonary tuberculosis) but infection may extend to brain, bones, eyes and skin (extrapulmonary tuberculosis) especially in HIV-infected patients. Tuberculosis Clinical picture of leprosy: Antitubercular agents • Based on history of discovery, sulfanilamide then dapsone then streptomycin (a turning point) then PAS then isoniazid then ethambutol then rifampin. • Due to multiple drug resistance, a combination of two or three of these agents is usually used. • Classification: A) p-Aminosalicylic acid derivatives. B) Pyridine carboxylic acid derivatives. C) Miscellaneous agents. COOH p-Aminosalicylic acid (PAS): OH Mode of action Two possible mechanisms: NH • Similar to the mode of action of sulfonamides: 2 Being structurally similar to PABA, it acts as a competitive antagonist to PABA by inhibiting COO the dihydropteroate synthetase eventually inhibits OH biosynthesis of mycobacterial DNA. • It chelates trace elements. Ca HN Disadvantages: O – Gastrointestinal irritation. – Short half life (short duration). • To overcome these disadvantages: – It should be formulated in enteric-coated dosage form or an antacid (aluminum hydroxide) is prescribed concurrently. – Developing less GI irritaing and longer acting 2 ++ Pyridinecarboxylic acid derivatives: Isoniazid (INH): O NHNH2 • Isonicotinic acid hydrazide. Synthesis: O OH N O OEt O NHNH2 OH EtOH / H2SO4 OH NH2NH2 OH N N N N Pyridoxine Mode of action: • Inhibition of the biosynthesis of mycolic acids (branched long chain fatty acids) which are important components of the cell wall of mycobacteria. • Active only against dividing mycobacteria. NOTES: 1) INH is the most active antitubercular agent rather than any other synthetic or antibiotic. 2) the equation of the assay is as follows: CONHNH2 N + 2Br2 + H2O COOH N2 + HBr + N 3)Ftivazide is hydrazone results from reaction of INH with benzaldehyde derivatives, It is as active as INH but non toxic. OCH3 CONHN C H Ftivazide N OH Side effects of INH: long-term therapy may result in fatal drug induced hepatitis. It causes peripheral neuritis as it results in pyridoxine deficiency by acting as a pyridoxine antagonist therefore, the concurrent administration of pyridoxine (vitamin B6) prevents the occurrence of peripheral neuritis. INH is metabolized by acetylation. Nearly half of the population are fast acylators of INH and the other half are slow acylators of INH. Slow acylators (including middle easterns) are genetically deficient of N-acetyltransferase and are more prone to the side effects of INH. S NH2 Ethionamide: Developed as a less toxic (5 times) analogue of INH. Mode of action like INH. Active only against dividing mycobacteria. N a SAR: H O N H b N H c Aromatic ring: N • If replaced by benzene, piperidine or thiazole loss of activity. Hydrazide moiety: • The -position is the best position for activity. • Replacement of Ha with alkyl groups decreases activity. • Replacement of Hb and/or Hc with small alkyl groups increases activity. large alkyl groups decreases activity. • Replacement of Hb and Hc with alkylidene Diazine derivatives ( pyrazinamide ). NH2 N O N Pyrazinamide N KMnO4 N / -CO2 N N quioxaline benzopyrazine Assay: COOH Pyrazinedioic acid NH2 N COOH N N Kjeldahl method COOH N CH3OH/HCl COOEt N NH3 N CONH2 N O N COONa + NaOH Boiling in AMMONIA + NH3 which is recieved in N distillation apparatus N boric acid to form ammborate which is titratrated by st HCl and methyl red indicator Miscellaneous agents Ethambutol (Etibi): Mode of action: • Being structurally similar to cellular polyamines (spermidine and spermine), it interferes with their function (essential for integrity of nucleic acids). H2N N H NH2 Spermidine • It chelates divalent metals thus inhibiting essential enzymes. O N H M O N H Synthesis of ethambutol: HO 2 NH2 + Cl Cl Base - 2HCl Antitubercular antibiotics: Streptomycin Cycloserine Rifampin Ethambutol Antileprotic agents A) Sulfones: O H2N S NH2 O Dapsone:Di-(4-aminophenyl)sulphone. • The drug of choice for the treatment of leprosy. • In addition to the antileprotic effect, dapsone has also antimalarial and antileshmanial activities. Mode of action: • Acts by a mechanism similar to sulfonamides. Evidences: • PABA partially antagonizes the action of sulfones. • Cross resistance between sulfonamides and sulfones. Disadvantage: • Poor solubility poor formulation Dapsone prodrugs (soluble dapsone analogs): Solapsone:Tetrasodium salt of Bis[4-(3phenyl-1,3disulphopropylamino)phenyl]sulphone O SO3Na HN S NaO3S NH O SO3Na NaO3S Solapsone undergoes acidic hydrolysis in the stomach to release dapsone which is the active antileprotic drug. Synthesis of Dapsone 2 Cl NO2 Na2S O2N S NO2 OXID K2Cr2O7 Dapsone RED Zn / HCl O2N O S O NO2 Synthesis of Solapsone O H2N S O NH2 condensation with 2 cinnamaldehyde H C H C O S O N CHO N 4NaHSO3 SO3Na NaO3S HN O S O NaO3S Solapsone NH SO3Na SAR of sulphones ( dapsone): 1) of the six isomers of diaminodiphenylsulphones only 4,4- is the active one. 2) additional substitution of phenyl ring cause loss of activity. 3)N-alkyl and N-acyl derivatives retain activity due to deacylation and dealkylation in vivo. 4)replacement of aminophenyl group with alkyl or aryl or heterocyclic group will cause loss in activity. 5)activity is exhibited by several mono and bis Schiffs base of aromatic aldehydes and aldehyde-bisulphite complexes as in glucosulphone, and glucosulphone has weak activity in vitro as no hydrolysis which regenerate ACTIVE DAPSONE. 6) sulphone group is essential for activity. B) Miscellaneous agents: Clofazimine:N,10-bis-(4-chlorophenyl)-2,10-dihydro-2[1-methylethylamino]-3-phenazinamine • A phenazine dye • Binds preferntially to mycobacterial DNA • Teratogenic Cl Thiambutosine: N N N N H Cl • A thiourea derivative • N-(4-butoxyphenyl)-N- -(4dimethylaminophenyl)thiourea BuO N S N H Other agents used for leprosy: • Isoniazid, ethionamide, rifampin N H The American Medical Association “AMA” states: • Treatment of leprosy is difficult and complex. • A combination of dapsone / clofazimine / rifampin should be used. • Therapy duration = 5 years – life time.