Case presentation
advertisement

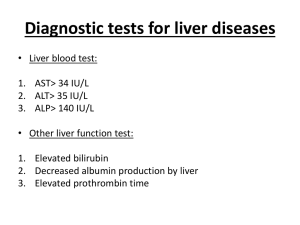
Evidence-Base Medicine Supervisor 施相宏 醫師 Intern 朱能生 李涵蓉 郭欣慧 99/12/23 Patient Profile • • • • • Chart No. :00042534 Name: 黃o崴 Age: 13 y/o Gender: male Admission date:99.11.16 Chief Complaint Fever up to 38。C this morning and skin rashes over face, extremities and trunk with itching sensation (since 11/16) Present illness-1 This 12-year-6-month old boy has suffered from folliculitis for almost a month. Because of the folliculitis, he went to the local clinics and has taken some medicine for almost a month. Present illness-2 Three weeks ago, he suffered from fever without any other symptoms. Last week, he suffered from fever again accompanied by skin rashes over extremities and abdomen. 11/15 - He vomited without other symptoms Present illness-3 11/16 - He suffered from fever at the third time with skin rashes over face, extremities and trunk, so he was brought to our emergency department for help. There were no cough, no rhinorrhea, no vomiting, no diarrhea and no tea color urine. Past history Systemic disease: denied Heart/liver disease(-),asthma(-) Hospitalization hx: 88-05-21 to 88-05-25 for hand-foot-mouth disease 89-10-4 to 89-10-9 for herpangina Vaccination: as scheduled Operation history: denied Full-term, delivered by : C-section Personal History Occupation: nil Travel history: nil Contact history: nil Allergy: denied drug or food allergy Current Medication Doxycycline Clindamycin Cotrizine Diclofenac Physical Examination • Consiousness: – alert • Vital sign: – – – – BP: 144/73 mmHg, PR: 80 bpm, RR: 16 cpm, BT: 37.4 ℃ • Head: • – – – – • Chest: – Breathing sound: coarse – Heart sound: regular heart beat, no murmur • Abdomen: . – – – – Soft and flat ,no tenderness Bowel sounds: normoactive Percussion: not tympanic Muscle garding (-) Conjuctiva: not pale Sclera: not icteric. Throat: no injected • Extremities Tonsil: no enlarged and congested – Free movable, no pitting edema Neck: – Supple, – No palpable lymphoadenopathy• Skin – No jugular vein engorgement – skin rash over face, chest, abdominal and extremities 99/11/16 : Lab data-1 99/11/16 : Lab data-2 99/11/16 : Lab data-3 Tentative diagnosis Acute liver injury Urticaria Proteinuria Folliculitis Management on 11/16 General survey (blood exam, urine routine , mycoplasma titer). IVF supply. Monitor vital sign and clinical s/s. Oral medications for symptoms relief. Arrange abdominal echo. Add cisbile use. After admission 99.11.16 (D1) Abdominal echo: 1.Increased liver echogenecity , susp. fatty liver or chronic liver, parnechymal disease. 99.11.19 (D3) 99.11.22 (D7) MBD 99.11.26 (D11) OPD 99.12.04 (D19) OPD GOT/GPT :178/1029 GOT/GPT : 63/ 391 ; r-GT:129 Bil (T/D):0.73/0.21 GOT/GPT : 33/ 139 ; r-GT:104 Bil (T/D): GOT/GPT : 30/ 52 ; r-GT:71 /0.28 Bil (T/D):0.85/0.23 GOT GPT T-Bil D-Bil Background question Question 1: How to approach the patient with suspected drug induced liver injury(DILI)? Question 2: How to treat DILI? Question 1~ How to approach the patient with suspected drug induced liver injury(DILI)? key diagnostic elements the time to onset clinical features the time and course of recovery specific risk factors the exclusion of other diagnoses previous reports on the hepatotoxicity of the implicated agent rechallenge liver biopsy Robert J. Fontana et al, Standardization of Nomenclature and Causality Assessment in Drug-Induced Liver Injury: Summary of a Clinical Research Workshop. HEPATOLOGY 2010;52:730-742. Clinical guidelines for DILI 1 Apply to our patient Careful history taking / rule out other etiologies Doxycycline, Clindamycin, Diclofenac, Ketoconazole for 2-3 wk herbal remedies(-), 鈣片(+) Drinking(-), smoking(-) underlying liver disease(-), other systemic disease (-) 10月中學校體檢肝指數正常 Rule out autoimmune hepatitis Rule out virus hepatitis Rule out bile duct disorders N ENGL J MED 354;7 Evaluation the type of liver injury 11/16 data AST = 1456 IU/L, ALT = 1586 IU/L , ALP = 775 IU/L R= (1586/45)/(775/495)= 22.51 heptocellular type UpToDate® Situations in which DILI should be particularly suspected: 1) start of a new drug in the past 3 months 2) presence of rash or eosinophilia 3) mixed type 4) cholestasis with normal hepatobiliary imaging and 5) acute or chronic hepatits without autoantibodies or hypergammaglobulinemia 2 Apply diagnostic scale CIOMS/RUCAM has been widely used as a standardized scale with high reliability, reproducibiliy and specificity RUCAM: 8 M&V: 16 3 Question 2 ~ How to treat DILI? Data source: Robert J. Fontana et al, Standardization of Nomenclature and Causality Assessment in Drug-Induced Liver Injury: Summary of a Clinical Research Workshop. HEPATOLOGY 2010;52:730-742. Discontinue the implicated drug as soon as diagnosis is suspected Corticosteroids, ursodiol and antioxidant therapy used in severe or progressive liver injury data supporting safety and efficacy are lacking N-acetylcysteine should be offered to patients with ALF due to DILI Apply to our patient Discontinue Doxycycline, Clindamycin, Diclofenac Supportive care Lab: ALT: 1586(11/16) -> 391(11/22) ALP: 775 (11/16) -> 648(11/22) 提出Foreground questions What are the prognostic factors of long-term outcome of patients with idiosyncratic drug-induced liver injury (DILI)? PICOT P Patients with a diagnosis of idiosyncratic druginduced liver injury with jaundice I Any factor that affects the long term outcome C Patient with DILI developed liver-related mortality and morbidity O Liver-related morbidity and mortality during the follow-up T Not defined The "5S" levels of organisation of evidence from healthcare research Brian Haynes, R Evid Based Med 2006;11:162-164 UpToDate DynaMed ACP PIER BMJ Clinical Evidence ACP journal club Evidencebasedmedic ine.com Cochrane Library BMJ Evidence Updates Other Systemic reviews eg. PubMed systemic reivew PubMed SUMsearch TRIP Google Copyright ©2006 BMJ Publishing Group Ltd. Search for useful information Data base : UpToDate, DynaMed, PubMed Key words: idiosyncratic, drug-induced liver injury, jaundice … should be followed by serial biochemical measurements. ... Recovery should be expected in the majority of patients after discontinuing the drug. … acute liver failure caused by an idiosyncratic drug reaction has a mortality rate of over 80 percent without liver transplantation. … recover from acute DILI with jaundice generally have a favorable prognosis, although some patients appear to develop progressive chronic liver disease. Drugs and the liver: Patterns of drug-induced liver injury Last literature review version 18.3: 九月 2010 This topic last updated: 七月 16, 2010 No meaningful finding! Search Studies, PubMed Title: The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice Einar Björnsson, Loa Davidsdottir Journal of Hepatology 50 (2009) 511–517 Introduction In general, the outcome of severe DILI has been considered an all-or-nothing phenomenon. Only a very few studies have analyzed the longterm outcome of patients with DILI. Although recent two studies have reported that chronicity following DILI is common, it has not been convincingly demonstrated that the perceived chronic liver injury does indeed lead to any liverrelated morbidity or mortality in the long run. Aithal PG, Day CP. Gut 1999;44:731–735. Pachkoria K, et al. Hepatology 2006;44:1581–1588 Patients and methods A cohort of patients with a diagnosis of DILI reported to the SADRAC 1970-2004 Only idiosyncratic DILI with jaundice (Bil≧ 2* ULN) were included (exclude acetaminophen induced DILI) The Fisher exact test for differences between groups regarding dichotomous variables; the Mann–Whitney test for continuous variables. All tests were two-tailed and were conducted at 5% significance level. SADRAC: Swedish Adverse Drug Reactions Committee *Cryptogenic cirrhosis: no known cause for the liver cirrhosis (include the metabolic syndrome) median follow-up of 11 yrs (range: 3–23 yrs) Results (1) Results (2) None of these patients were followed to complete normalization of liver tests during the original evaluation for the DILI. Results (3) 6/7(86%) pts with protracted DILI were CS type. 5/685(0.73%) pts were diagnosed with AIH during f/u, and all were female gender. Results (4) In three out of five cases who developed AIH, the immunosuppressive treatment could be discontinued at follow-up. Results (5) Pts with a liver-related morbidity and mortality during the follow-up had been treated with the drug associated with DILI for a longer period of time (135 ± 31 days vs. 53 ± 3 days, p < 0.0001) Discussion (1) Development of clinically important liver disease is rare (23/685, 3.4%) when a patient has survived a severe DILI. Protracted DILI was mostly seen in patients with cholestatic/mixed type (6/7, 86%) of DILI in whom liver tests in the vast majority of cases normalized during follow-up. Autoimmune hepatitis (5/23, 22%) developed in a substantial number of patients with a seemingly good prognosis. Ortiz N, et al. J Hepatol 2008;48:A896. Yamamoto S. J Gastroenterol 2002;37:1345–1346 Discussion (2) Duration of drug therapy before diagnosis of DILI was found to be significantly longer in those who experienced liver-related morbidity/mortality during follow-up. Decompensated ‘‘cryptogenic” cirrhosis developed in some patients in which DILI might have played a role in this development. Appraisal AAMPICOT Answer: Does this paper answer your question? Yes. Author: Is the author an expert of the field? yes Is there any conflict of interest? Not mentioned Method Level of evidence Level Prognosis 1a SR (with homogeneity) of inception cohort studies; CDR validated in different populations 1b Validating cohort study with good reference standards; or CDR tested within one clinical centre 1c All or none case-series 2a SR (with homogeneity) of either retrospective cohort studies or untreated control groups in RCTs 2b Retrospective cohort study or follow-up of untreated control patients in an RCT; Derivation of CDR or validated on split-sample only 2c "Outcomes" Research 3a 3b 4 Case-series (and poor quality prognostic cohort studies) 5 Expert opinion without explicit critical appraisal, or based on physiology, bench research or "first principles" Grades of Recommendation A consistent level 1 studies B consistent level 2 or 3 studies or extrapolations from level 1 studies C level 4 studies or extrapolations from level 2 or 3 studies D level 5 evidence or troublingly inconsistent or inconclusive studies of any level PICOT P Patients with a diagnosis of idiosyncratic druginduced liver injury with jaundice I Any factor that affects the long term outcome C Patient with DILI developed liver-related mortality and morbidity O Liver-related morbidity and mortality during the follow-up T Not defined PROGNOSIS WORKSHEET (VIP rule) Are the results of this prognosis study valid? Was a defined, representative sample of patients assembled at a common (usually early) point in the course of their disease? Yes.(662/685 pts diagnosed with DILI within a mean of 53 ± 3 days) 2.Was patient follow-up sufficiently long and complete? Yes. (median follow-up: 11 yrs, range: 3–23 yrs). 3.Were objective outcome criteria applied in a “blind” fashion? No. (Lab data and hx all need to be measured or asked.) 4.If subgroups with different prognoses are identified, was there adjustment for important prognostic factors? Yes. (But it should be very careful) 5.Was there validation in an independent group (“test-set”) of patients? No. (There was no such independent group set up for this study.) 1. Are the valid results of this prognosis study important? How likely are the outcomes over time? Not sure. (But it may play a role while physicians meet the pts with DILI) 2.How precise are the prognostic estimates? Not sure. (It needs to be used in different races to confirm these results.) 1. Can you apply this valid, important evidence about prognosis in caring for your patient? Were the study patients similar to your own? Not exactly, due to our pt is a teenager not an adult. 2.Will this evidence make a clinically important impact on your conclusions about what to offer or tell your patient? Yes. 1. Apply 醫療現況 病人已順利出院,改由門診追 蹤。 病人意願 未知,但其與家屬在臨床醫療 的配合度佳。 生活品質 社會脈絡 由於患者於診斷DILI前用藥天數 約一個月,單純就文獻結果而言, 應屬預後較差的一群,故有賴良 好習慣的維持與長期追蹤,以確 保終身生活品質。 只要患者的狀況能有效改善,且 無complication或危害生活品質, 適當地衛教加上長期追蹤,仍是 最值得推展的醫療方針。 Audit 在「提出臨床問題」方面的自我評估 我提出的問題是否具有臨床重要性?是,可作為預後參考 我是否明確的陳述了我的問題? 我的foreground question 是否可以清楚的寫成PICO?可 我的background question是否包括what, when, how, who等字根?未能全部涵蓋 我是否清楚的知道自己問題的定位?(亦即可以定位自己 的問題是屬於診斷上的、治療上的、預後上的或流行病學 上的),並據以提出問題?知道,屬於預後範疇。 對於無法立刻回答的問題,我是否有任何方式將問題紀錄 起來以備將來有空時再找答案? 有,將其另列問題清單 在「搜尋最佳證據」方面的自我評估 我是否已盡全力搜尋?是 我是否知道我的問題的最佳證據來源?是 我是否從大量的資料庫來搜尋答案?是 我工作環境的軟硬體設備是否能支援我在遇到問 題時進行立即的搜尋?尚可,學校的電子資源仍 有不足之處 我是否在搜尋上愈來愈熟練了?是 我會使用「斷字」、布林邏輯、同義詞、MeSH term,限制(limiters)等方法來搜尋?是 我的搜尋比起圖書館人員或其他對於提供病人最 新最好醫療有熱情的同事如何?應屬「刻苦耐勞型」 關於「嚴格評讀文獻」方面的自我評估 我是否盡全力做評讀了?是,已盡力而為 我是否了解Number need to treat 的意義?了解 我是否了解Likelihood Ratios的意義?了解 我是否了解worksheet每一項的意義?了解 評讀後,我是否做出了結論?是 關於「應用到病人身上」的自我評估 我是否將搜尋到的最佳證據應用到我的臨床工作 中?目前尚無機會,但未來可運用 我是否能將搜尋到的結論如NNT,LR用病人聽得懂 的方式解釋給病人聽?能 當搜尋到的最佳證據與實際臨床作為不同時,我 如何解釋?臨床工作不一定能面面俱到,且人種 與國情差異也需考量 改變「醫療行為」的自我評估 當最佳證據顯示目前臨床策略需改變時, 我是否遭遇任何阻止改變的阻力?沒有 我是否因此搜尋結果而改變了原來的治療 策略?做了那些改變?未改變,因搜尋結果為 預後相關,不影響原來治療策略,但可能影響後 續追蹤時的臨床決策。 效率評估 這篇報告,我總共花了多少時間? 2天 我是否覺得這個進行實證醫學的過程是值 得的?值得,因為對臨床工作有極高的參考價值 我還有那些問題或建議?目前暫無 Thanks for your attention!