pah_presentation2
advertisement
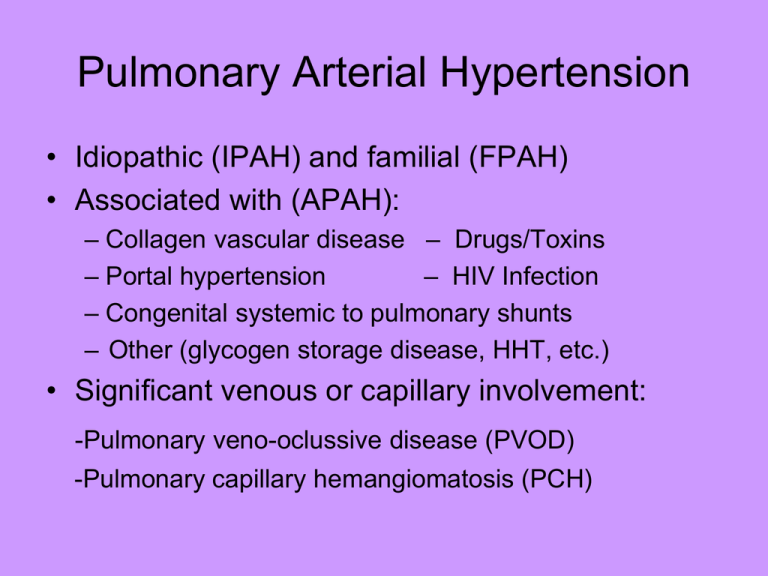
Pulmonary Arterial Hypertension • Idiopathic (IPAH) and familial (FPAH) • Associated with (APAH): – Collagen vascular disease – Drugs/Toxins – Portal hypertension – HIV Infection – Congenital systemic to pulmonary shunts – Other (glycogen storage disease, HHT, etc.) • Significant venous or capillary involvement: -Pulmonary veno-oclussive disease (PVOD) -Pulmonary capillary hemangiomatosis (PCH) Goals of Patient Evaluation • Determine presence of PAH • Determine cause of PAH (primary or secondary) *IPAH is diagnosis of exclusion • Determine severity of PAH *Impact on patient’s life and overall wellbeing. • Determine functional status of patient • Determine treatment modality appropriate for patient (support system, tolerance of PAH, mental capacity, etc) Presenting symptoms in PAH Common Initial Symptoms (N=187) Patients (%) Dyspnea 60 Fatigue 19 Syncope or near syncope 13 Chest pain 7 Palpitations 5 Leg edema 3 Vascular Pressure in Systemic and Pulmonary Circulations (mm Hg) Systemic Circulation 120/80, mean 90 25/10, mean 15 Arteries Arteries Right Atrium Mean >6 Left Atrium Mean 5 Pulmonary Circulation Body SVR= 17.6 Veins Right Ventricle 25/5 Left Ventricle 120/5 PVR= 1.8 Veins Role of ET-1 and Its Receptors Are Important in PAH ET-1 is elevated in PAH: Correlates to disease severity • Regulated by ETA and ETB receptors – ETA: vasoconstriction, cell proliferation – ETB: vasodilation, antiproliferation (increased NO and PGI² production) Pulmonary circulation • Low resistance, high compliance vascular bed • Only organ to receive entire cardiac output (CO) • Changes in CO as well as pleural/alveolar pressure affect pulmonary blood flow • Different reactions compared to the systemic circulation-hypoxia • Normally in a state of mild vasodilation ETA Receptor Pathway ETB Receptor Pathway The Common Denominator of PAH: Pulmonary Vasculopathy Intimal thickening due to endothelial proliferation Medial thickening and muscularization Plexiform lesions The Hemodynamic “Working” Definition of Pulmonary Arterial Hypertension • Systolic pulmonary arterial pressure 35 to 40 mm Hg • Mean pulmonary arterial pressure ≥25 mm Hg • PWP, LAP, LVEDP <15 mm Hg • Pulmonary vascular resistance >3U PCWP=pulmonary capillary wedge pressure; LAP=left arterial pressure; LVEDP=left ventricular end-diastolic pressure. Diagnostic Classification of Pulmonary Hypertension (PH)* • Group 1. Pulmonary arterial hypertension • Group 2. Pulmonary venous hypertension • Group 3. PH associated with disorders of the respiratory system and/or hypoxemia • Group 4. PH due to chronic thromboembolic disease • Group 5. Miscellaneous 3rd world symposium on pulmonary arterial hypertension, JACC 2004 Normal Hemodynamics I. Normal parameters • Cardiac output = 5-6l/m for men • Normal pressures for different cardiovascular compartments: • RA 0-5mmHg • RV syst 25mmHg; diast 0-5mmHg • PA syst 25mmHg; diast 10mmHg • PCWP 10mmHg • LA 10mmHg • LVsyst 120mmHg diast 10mmHg • Aorta syst 120mmHg diast 80mmHg Optional Tests in PAH diagnosis • • • • • Sleep study Pulmonary angiography Exercise physiology study Evaluation for coronary artery disease Transesophageal echocardiography for valvular heart disease, shunt PAH May Occur at All Ages: Distribution of Patients by Age More frequent in women than men-2.5 to 1 30 Males Females 25 20 Frequency (%) 15 10 5 0 18-20 21-30 31-40 41-50 51-60 Age (y) 61-70 >70 NYHA/ WHO Functional Class in assessment of PAH • Class I: Minimal symptoms, no activity limitation • Class II: Dyspnea or fatigue with ordinary activity • Class III: Comfortable at rest only; dyspnea, fatigue, chest pain or near syncope with minimal activity • Class IV: Dyspnea and/or fatigue at rest and/or signs of right heart failure Determining Therapy • Based on severity of illness: • 6 minute walks are extremely important to determine functional limitations, response to therapy. -patient symptomology is paramount, if syncopal or s/sx of heart failure, regardless of current hemodynamics, use FLOLAN, unless no social support, patient mentally impaired or refuses this therapy. (Current philosophy among PH specialists is that it is better to treat with the best therapy available, then back down to less invasive therapy) -Hemodynamic parameters (RAP, PAP Mean, COP, CI, PVR, PA O2 sat) secondary -Exercise tolerance (NYHA Functional class) Pulmonary Arterial Hypertension: Goals of Therapy • Improve exercise capacity with decrease in symptoms • Improve functional class • Prevent clinical worsening • Improve survival • Improve hemodynamics Conventional Therapy • Anticoagulation (coumadin, warfarin) • Diuretics (lasix, aldactone, zaroxolyn) • Calcium Channel Blockers (cardizem, procardia, norvasc), only if >20% decrease to <40 mmHg with vasodilator challenge • Oxygen therapy Basic Treatment: Anticoagulation • Recommended for patients with IPAH – benefit not shown in other groups with PAH • Studies only in IPAH patients: – benefit demonstrated 3 studies – neither randomized, 2 retrospective • No evidence for effect on disease • Not likely to affect symptoms • Suggested INR 1.5-2.5, unless CTEPH: 2.5-3.5 • Catheter prophylaxis INR 1.5-2.2 Basic Treatment : Diuretics • Reduce peripheral edema, intravascular volume, and central venous pressure • Combination of loop diuretic (furosemide (lasix), Bumetanide (bumex), torsemide (demadex) /spironolactone (aldactone),/ metolazone may be beneficial • IV diuretics in refractory cases • Can significantly improve symptoms and function • Titrate to keep patient edema free or until BUN/Cr elevate • Low BP is not a contraindication to diuretics Calcium Channel Blockers (CCBs) • Largest prospective study1: – 64 pts w/IPAH Rx with CCB, followed for up to 5 yrs – ave. dose: nifedipine - 172 41mg, diltiazem - 720 208 mg – 17 pts (26%) responded to Rx with 39% in mPAP and 53% in PVR • Recent retrospective study of 557 pts w/IPAH showed only 6.8% long-term response with CCBs1, lower in other PAH groups (secondary) • If patient is a responder, but does not tolerate one type of CCB, may do well with another type of CCB 2. Rich et al, NEJM, 1992 1. Sitbon et al, Circulation, 2005 Clinical Therapies Available • • • • • • • • • Flolan® (epoprostenol) continuous infusion Tracleer® (bosentan) Remodulin® (treprostinil) – SQ and IV Ventavis® (iloprost) Revatio® (sildenafil) Letairis® (ambrisentan) Atrial Septostomy Lung Transplantation Pulmonary Thromboendarterectomy (UCSD, Vanderbilt, Cleveland Clinic) • Septal Defect Closure Devices Flolan • • • • • • • Approved for functional class III-IV Given via continuous infusion with mechanical pump Short half life of 3 to 6 minutes Must be kept cold for maximum effectiveness Requires implanted central venous catheter Must do daily sterile mixing of the medication Needs nursing support for up-titration, medication related problems or catheter issues • Requires intensive education prior to and during initiation of therapy • Must have insurance approval prior to elective initiation • Consider referral for lung transplantation if no significant improvement with therapy CADD 1 Legacy Pump • Easy to read and understand display. Easy to operate with sufficient training. • Battery Powered • Portable, weighs about 7 pounds with full cassette and ice packs. • Not waterproof • Pump sensitive to extreme temperatures Flolan protocol • • • • • • • • • • • • Provide PH flolan patient/family education booklet for review/possible questions. If patient agreeable, proceed. Send referral form to Accredo Therapeutics, must receive approval prior to initiation of therapy (clinical records, procedure results, CCB statement, sleep study, if any, medical necessity for flolan, etc) Once patient approved, order start kit for flolan therapy (pumps x 2, pouch, ice packs, batteries, educational material) Schedule pre-hospital teaching (1-2 days) with Accredo nurse clinical specialist Schedule patient admission and reserve telemetry bed Schedule Hickman catheter placement or PICC if emergent start or unsure therapy will be effective. No port-a-caths for flolan. Coordinate with pharmacy for dispensing of flolan, diluent, medication cassettes and tubing for entire patient hospitalization Once patient admitted, begin intensive patient education (approx 4 hours per day). Provide practice supplies for patient and family once nurse time completed. Encourage them to PRACTICE, PRACTICE, PRACTICE mixing Document progress, flolan increases, side effects, arrhythmias, increase activity to assess effects of flolan Arrange Home Health RN for 3 to 4 days after discharge from hospital f/u in clinic with PH specialist in one month, CM will schedule Call nurse case manager weekly for flolan increases, side effects, issues Flolan Patient Education • Flolan -mix and administration of medication cassettes (back-up daily) -anticipated dose increase schedule – daily while hospitalized then once or twice weekly after discharge to home based on side effects -programming of CADD 1 Legacy pumps -Hickman catheter care and maintenance including dressing changes and monitoring for infection -Emergency management/EMS letter • 2 Gm sodium diet restriction/2000 cc fluid restriction • Coumadin management and drug interactions • Quick reference for managing flolan • Cold and Flu season management • Patient f/u schedule: clinic evaluation at 1 month, echo and CXR at 4 months, cath at 1 year. • Managing other co-morbid illnesses – PCP involvement Bosentan (Tracleer) • Oral, non-selective ET-1 receptor antagonist • Bosentan (2 doses) vs. placebo evaluated in a multicenter center (Breathe-1) study • 213 PAH patients evaluated over 16 weeks: -70% IPAH, 30% CTD -90% class III, 10% class IV • Primary endpoint: change in 6 min walk distance - showed an increase in exercise tolerance with walk distance increase of 30 to 40 meters. Rubin et al NEJM, 2002 Use of bosentan • Approved for treatment of Functional Class III and IV PAH • Initiate at 62.5 mg bid x 4 wks, then increase to 125 mg bid. For patients < 40 kg body weight, start at 31.25 mg. • Need to follow monthly LFTs (generally observe increase in first few months of use, but may occur years later) • Dose reduction necessary for significant elevations in liver enzymes (3 to 5 times above normal) • Teratogenic (causes birth defects) in animals, need to check beta-HCG (pregnancy test) at BL and q monthly • Can cause transient anemia (10 point drop in hematocrit), so need to monitor CBC every 3 months. The Common Denominator of PAH: Pulmonary Vasculopathy Intimal thickening due to endothelial proliferation Medial thickening and muscularization Plexiform lesions Treprostinil (Remodulin) • Longer acting prostacyclin analogue, given sq or IV by continuous infusion - half-life 4 hours • SQ Continuously infused via a CADD MS 3 pump; IV via CADD 1 Legacy or Chrono 5 • Evaluated SQ in a 12 week multi-center, placebo-controlled study of 471 patients with PAH1: -IPAH, CTD and CHD -NYHA class II-IV (81% class III) • Primary endpoint: change in six-minute walk distance-walk test improved 34 meters at 4th quarter interval 1. Simonneau et al. AJRCCM, 2002 Treprostinil - Summary • Approved for treatment of Functional Class II, III and IV PAH (SQ and IV) • At higher doses, there may be more significant benefits. Many times patients require two to three times as much treprostinil as flolan. • Majority of patients experience pain or discomfort at the infusion site with SQ therapy (8% D/C rate during the study) CADD MS 3 Ambulatory Infusion Pump • Drug and Pump for SQ use shown below-IV Drug is the same, pump is the CADD 1 Legacy or Chrono 5 Pump. Remodulin Protocol • • Discuss therapy with patient and distribute patient education material If patient agreeable, submit remodulin referral to Accredo, Curascript, or Caremark. • Once approval obtained, order start kit from distributor to be shipped to clinical specialist for pre-initiation patient education. Remind patient to bring start kit with them to hospital on day of initiation. • Arrange date for pre-initiation teaching. • For SQ Remodulin, reserve clinic room and schedule nurse clinic visit -Patient education: drug, use of reservoir, programming of pump, battery change, site rotation, managing side effects, reordering of supplies. Discuss weekly increases as tolerated. -f/u in clinic at 3 month intervals, echo at 6 months, cath at one year. • For IV remodulin, reserve telemetry bed for patient admission and schedule Hickman catheter placement. -follow flolan protocol with regard to patient education. -HH nurse for 2 to 3 visits after discharge from hospital for ongoing patient education. - f/u in clinic at 1 month, then 3 month intervals with echo at 6 months, cath at 1 year. **Consider additional therapy if no significant benefit at 3 months or disease progression. Iloprost (Ventavis) • • • • • • Prostacyclin analogue with half-life of ~45-60 min Can be given by inhalation (or intravenously) Evaluated in randomized, multi-center (AIR) study1 203 patients: 72% w/IPAH, 28% w/CTEPH Functional class III (59%), class IV (41%) Combined primary endpoint: – 10% increase in 6 min walk distance after inhalation – improvement in functional class – No clinical deterioration or death 1.Olschewski et al. NEJM, 2002 Adaptive Aerosol Delivery (AAD®) • Analyzes pressure changes relevant to flow of first 3 breaths • Delivers aerosol during first phase of inspiration • Continually monitors and adapts to individual changes in breathing pattern Ventavis-Summary • Iloprost approved for treatment of class III and IV PAH patients • Well tolerated by most patients • Less invasive than other prostacyclin therapies • Requires 6-9 inhalations/D, 7 to 15 min/Trt • No benefit in patients with CETPH • Will be used mainly in combination with oral therapy in USA Ventavis I-neb Only the I-neb and Pro-dose have been proven to deliver safe and accurate dosing of Ventavis. While the Pro-dose system requires availability of electricity, with the I-neb, a patient can get up to 40 treatments with a single charge, giving them freedom and flexibility. CoTherix is looking into alternatives for medication delivery, including changes to the existing I-neb, as well as the possibility of a powdered form of Iloprost. Ventavis protocol • • • • • Discuss therapy with patient and distribute patient education material If patient agreeable, submit referral form to Accredo or Curascript for Ventavis along with required documentation (same as with flolan) Once patient approved for therapy, arrange pre-initiation education with community clinical specialist. Schedule patient for nurse clinic visit (requires approximately 4 hours) Reinforce patient education done at patient’s home: - Drug actions, side effects, management of side effects, s/sx to report to case manager or PH specialist, placement of medication in and cleaning of nebulizers, reordering of supplies, and calling distributor for nebulizer issues. • • • • • • • • • Take baseline VS, O2 sats Patient completes 1st treatment of 2.5 mcg by inhalation VS and O2 sats repeated at 30 and 60 minutes, along with review of side effects. Wait an additional hour (treatments are 2 hours apart), then take baseline VS for 2nd inhalation Patient completes 2nd treatment of 5 mcg by inhalation VS and O2 sats repeated at 30 and 60 minutes, as well as review of side effects. Patient to perform 6 minute walk test after 2nd treatment to assess exercise tolerance and presence of side effects. f/u in clinic every 3 months, echo at 6 months, cath at one year. Consider additional therapy if no significant benefit at 3 months or progression of disease. Sildenafil • Multi-center, randomized, double blind, placebo controlled, 12 week trial (SUPER) • 280 patients enrolled, four treatment arms: – placebo – sildenafil at 20, 40 and 80 mg TID • 63% IPAH, 37% CTD, 7% CHD • 39% class II, 58% class III, 3% class IV • Primary endpoint: change in six-min walk distance Sildenafil (Revatio) • Sildenafil demonstrated significant improvement vs. placebo respect to: – 6MWD with all doses – Hemodynamic parameters – Functional class • Acceptable safety profile • FDA approved for functional class II, III and IV • Available at local pharmacies and specialty pharmacies. May need to obtain prior-authorization due to expense of therapy, approximately $1200/month. • Initiated at home with 10-20 mg po TID, may need to increase to 50 mg po TID, while obtaining insurance approval can be difficult. • f/u in clinic at 3 months, echo at 6 months, cath at 1 year • Consider additional therapy if no significant benefit at 3 months. Ambrisentan (Letairis®) • Oral endothelin-A selective blocking agent • Approved for treatment of Functional Class II and III PAH • Initiate at 5 mg daily x 4 wks, then increase to 10 mg daily long-term as tolerated. For patients < 40 kg body weight, start at 2.5 mg. • Need to follow monthly LFTs (lower incidence of elevations in LFTs noted in clinical trial than with Tracleer) • Dose reduction necessary for significant elevations in liver enzymes (3 to 5 times above normal) • Teratogenic (causes birth defects) in animals, need to check beta-HCG (pregnancy test) at BL and q monthly • Can cause transient anemia (10 point drop in hematocrit), so need to monitor CBC every 3 months. • Monitor closely for increased occurrence of edema Combination Therapy • Increasingly being used to treat PAH due to disease complexity (3 pathways: nitric oxide pathway, prostacyclin pathway, and endothelin pathway • Combinations: -Epoprostenol and bosentan (study completed) -Bosentan and iloprost (study completed) -Epoprostenol and sildenafil (on-going) -Treprostinil and bosentan (on-going) -Bosentan and tadalafil (on-going) -Bosentan and sildenafil (on-going) Investigational Medications • Sitaxsentan - endothelin A receptor antagonist, dosed once daily (definite interaction with coumadin) • Inhaled treprostinil - Administration of treprostinil medication performed by inhalation (6 to 12 puffs) with the OPTINEB™ ultrasonic nebulizer four times daily in combination with Tracleer or Revatio at stable dose • Oral treprostinil - oral remodulin in timereleased capsule with twice daily dosing (UT15C) • Tadalafil (Cialis) - phosphodiesterase inhibitor with once daily dosing Investigational Medications (cont): • Pulmolar-potent inhibitor of vascular smooth muscle and endothelial cell proliferation, markedly reduced vascular remodeling and right ventricular hypertrophy, and reduction of pulmonary hypertension and inflammatory cell infiltration in lung tissues: 2-methoxyestradiol (2ME), an endogenous non-estrogenic metabolite of estradiol. • Gleevec-used now to treat leukemia; The substance conveys its potent anti-proliferative effect by selectively suppressing the tyrosine kinase pathway. In cancer, tissue proliferation is uncontrolled and leads to the spreading of the tumor. In pulmonary hypertension, also, uncontrolled growth of the vascular wall is the underlying mechanism of the disease. Investigational Medications (cont): • Biomarin: Nitric Oxide Precursor (may be a good indicator of survival long-term • (Rho kinase inhibitors: Protein Kinase Inhibitors-Hydroxyfasudil) • REVEAL - Registry to EValuate Early And Long-term PAH disease management = National PAH Disease Registry launched in 2006 Comparison of Medical Treatments Cost $ (annual) Route Frequency Epoprostenol ~100,000 IV Continuous + +++ Yes Bosentan Ambrisentan ~48,000 Oral BID QD +++ + Yes No Treprostinil >150,000 SQ, IV Continuous ++ +++ Yes Iloprost 70-80,000 Inhaled 6-9X per day ++ ++ No Sildenafil ~15,000 Oral TID +++ + Yes Ease Side of Use effects Longterm Data