Chapters 1 and 2
advertisement
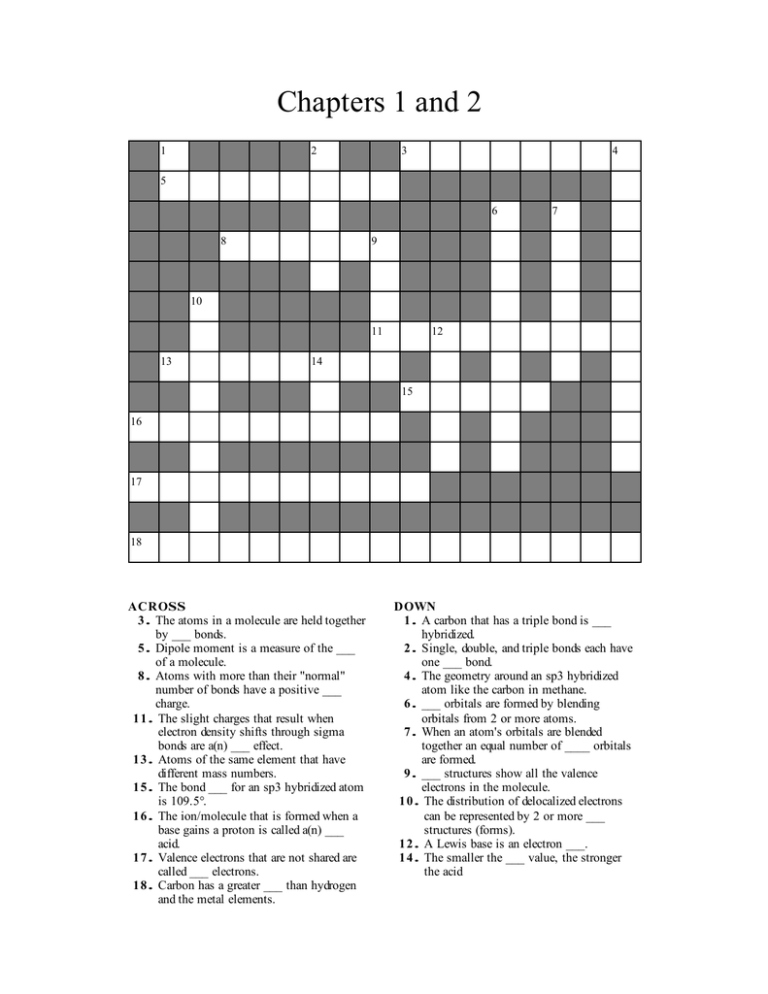
Chapters 1 and 2 1 2 3 4 5 6 8 7 9 10 11 13 12 14 15 16 17 18 ACROSS 3. The atoms in a molecule are held together by ___ bonds. 5. Dipole moment is a measure of the ___ of a molecule. 8. Atoms with more than their "normal" number of bonds have a positive ___ charge. 11. The slight charges that result when electron density shifts through sigma bonds are a(n) ___ effect. 13. Atoms of the same element that have different mass numbers. 15. The bond ___ for an sp3 hybridized atom is 109.5°. 16. The ion/molecule that is formed when a base gains a proton is called a(n) ___ acid. 17. Valence electrons that are not shared are called ___ electrons. 18. Carbon has a greater ___ than hydrogen and the metal elements. DOWN 1. A carbon that has a triple bond is ___ hybridized. 2. Single, double, and triple bonds each have one ___ bond. 4. The geometry around an sp3 hybridized atom like the carbon in methane. 6. ___ orbitals are formed by blending orbitals from 2 or more atoms. 7. When an atom's orbitals are blended together an equal number of ____ orbitals are formed. 9. ___ structures show all the valence electrons in the molecule. 10. The distribution of delocalized electrons can be represented by 2 or more ___ structures (forms). 12. A Lewis base is an electron ___. 14. The smaller the ___ value, the stronger the acid