Electron Configurations: Chemistry Presentation
advertisement

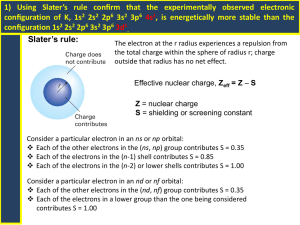
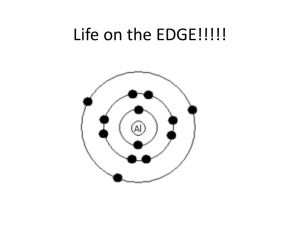
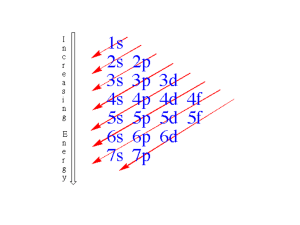
Electron Configurations • Of the three major subatomic particles, the electron plays the most significant role in determining the physical and chemical properties of an element. The arrangement of elements in the periodic table depends on these properties. • Thus, there should be some relationship between the electron configurations of the elements and their placement in the table. Electron Configurations • The orbital names s, p, d, and f stand for names given to groups of lines in the spectra of the alkali metals. These line groups are called sharp, principal, diffuse, and fundamental. Electron Configurations s p 1 2 3 4 5 6 7 d (n-1) f (n-2) 6 7 Electron Configurations • The electron configuration of an atom denotes the distribution of electrons among available shells. • The standard notation lists the subshell symbols, one after another. • The number of electrons contained in each subshell is stated explicitly. • For example, the electron configuration of beryllium, with an atomic (and electron) number of 4, is 1s22s2 or [He]2s2. Electron Configurations • C: 1s2 2s2 2p2 • Ne:1s2 2s2 2p6 • S: 1s2 2s2 2p6 3s2 3p4 or [He] 2s2 2p2 or [He] 2s2 2p6 or [Ne]3s2 3p4 s p 1 2 3 4 5 6 7 d (n-1) f (n-2) 6 7 Aufbau Principle • Electrons fill orbitals starting at the lowest available energy states before filling higher states (e.g. 1s before 2s). • The number of electrons that can occupy each orbital is limited by the Pauli Exclusion Principle (each orbital can hold two electrons with opposite spins). The rules of the Aufbau Principle are: “The Lazy Tenant Rule” • 1.Electrons are placed in the lowest energetically available subshell. • 2. An orbital can hold at most 2 electrons. • 3.If two or more energetically equivalent orbitals are available (e.g., p, d etc.) then electrons should be spread out before they are paired up (Hund's rule). Hund's Rule “The Empty Bus Seat Rule” • If multiple orbitals of the same energy are available, Hund's Rule says that unoccupied orbitals will be filled before occupied orbitals are reused (by electrons having different spins). WRONG RIGHT Increasing energy 7p 7s 6p 6s 5p 5s 4d 3d 3p 3s 2p 1s 5d 4p 4s 2s 6d 5f 4f The Noble Gases • These are the elements in which the outermost s and p subshells are filled. The noble gases have full outer shells; notice that these elements have filled outermost s and p sublevels • • • • Helium Neon Argon Krypton 1s2 1s22s22p6 1s22s22p63s23p6 [He] [Ne] [Ar] 1s22s22p63s23p63d104s24p6 [Kr] Representative Elements • In these elements, the outermost s or p sublevel is only partially filled. There are three groups of representative elements: • Group 1 alkali metals • Group 2 alkaline earth metals • Group 7 halogens Representative Elements • For any representative element, the group number equals the number of electrons in the outermost energy level (valence electrons) • Potassium 1s22s22p63s23p64s1 • Carbon, silicon, and germanium, in Group 4, have four electrons in the outermost energy level • Carbon 1s22s22p2 • Silicon 1s22s22p63s23p2 • Germanium 1s22s22p63s23p63d104s24p2 Transition Metals • These are metallic elements in which the outermost s sublevel and nearby d sublevel contain electrons. The transition elements are characterized by addition of electrons to the d orbitals Inner Transition Metals • These are the metallic elements in which the outermost s sublevel and nearby f sublevel generally contain electrons Write the electronic configurations for the following elements • • • • • • O Na Ar Fe Ca Ce 1s22s22p4 or [He]2s22p4 1s22s22p63s1 or [Ne]3s1 1s22s22p63s23p6 or [Ne]3s23p6 1s22s22p63s23p63d64s2 or [Ar]3d64s2 1s22s22p63s23p64s2 or [Ar]4s2 1s22s22p63s23p63d104s24p64d105s25p64f15d16s2 or [Xe]4f15d16s2 Half-Full and Full Subshells • full subshell: fully-filled shells are lower in energy than partially-filled shells (i.e. Noble Gases) • half-filled subshells: lower in energy than partially-filled subshells • Cu exception: [Ar] 4s13d10 rather than [Ar] 4s23d9 • Cr exception: [Ar]4s13d5 rather than [Ar]4s23d4