Document

Please select a Team.
1. Females
2.
Those that aren’t female
F em al es
0% 0%
T ho se
th at
a re
...
The order of elements in the modern periodic table is based on
1.
the number of protons in the nucleus.
2.
the electric charge of the nucleus.
3.
the number of neutrons in the nucleus.
0% 0% 0%
th e nu m be r of
..
.
th e el ec tr ic
c
...
th e nu m be r of
..
.
10
Atoms of elements that are in the same group are in the same
1. Horizontal row
2. Vertical column
H or izo nt al
ro w
0% 0%
V er tic al
c ol um
...
10
Atoms of elements that are in the same group have the same number of
1. protons.
2. neutrons.
3. valence electrons.
4. protons and neutrons.
0% 0% 0% 0%
p ro to ns
.
n eu tr on s.
v al en ce
e le ct ro ns
.
p ro to ns
a nd
n eu tr on s.
10
Which of the following elements is an alkali metal?
1. Magnesium (# 12)
2. Fluorine (#9)
3. Titanium (#22)
4. Lithium (#3)
5. Neon (#10)
0% 0% 0% 0% 0%
M ag ne si um
(#
1
...
F lu or in e
(#
9)
T ita ni um
(#
22
)
L ith iu m
(#
3)
N eo n
(#
10
)
Which of the following elements is an alkaline-Earth metal?
1. Magnesium (# 12)
2. Fluorine (#9)
3. Titanium (#22)
4. Lithium (#3)
5. Neon (#10)
0% 0% 0% 0% 0%
M ag ne si um
(#
1
...
F lu or in e
(#
9)
T ita ni um
(#
22
)
L ith iu m
(#
3)
N eo n
(#
10
)
Which of the following elements is a halogen?
1. Magnesium (# 12)
2. Fluorine (#9)
3. Titanium (#22)
4. Lithium (#3)
5. Neon (#10)
0% 0% 0% 0% 0%
M ag ne si um
(#
1
...
F lu or in e
(#
9)
T ita ni um
(#
22
)
L ith iu m
(#
3)
N eo n
(#
10
)
Semiconductors are elements that
1.
have large atomic masses but small atomic numbers.
2.
do not form compounds.
3.
can conduct heat and electricity under certain conditions.
4.
are extremely hard.
0% 0% 0% 0%
h av ic
m
...
e la rg e at om
d o no t f or m
c om
c po u.
..
an
c on du ct
h ea t a nd
...
a re
e xt re m el y ha rd
.
Carbon and other nonmetals are found in which area of the periodic table?
1. on the left-most side
2. on the right side
3. in the middle column of the periodic table
4. in the bottom rows
0% 0% 0% 0%
o n th e le ft e
-m os t s id
o n th e rig ht
s id e id dl e co
th e m
in
..
lu m n.
in
th e bo tt om
ro w s
10
500
500
480
480
480
Team 2
Team 4
Team 1
Team 3
Team 5
Team Scores
In Mendeleev’s periodic table, elements in each column had similar
1. Physical properties.
2. Chemical properties.
3. Both of these
4. None of these
0% 0% 0% 0%
...
P hy si ca l p ro pe
C he m ic al
p ro pe
...
B ot h of
th es e
N on e of
th es e
As you move from left to right across the periodic table, elements
1. become less metallic.
2. have a lower atomic number.
3. have a larger atomic radius
4. become more metallic.
0% 0% 0% 0%
b ec om e le ss
m e.
..
h av e a lo w er
a
...
h av e a la rg er
..
.
ec om
b e m or e m e.
..
What is the location of elements in the periodic table related to?
1. color
2. number of neutrons
3. atomic weight
4. electron configuration
0% 0% 0% 0%
c ol or
n um be r of
n eu tr on s
a
w ei gh t to m ic
e le ct ro n co nf ig ur at io n
10
Which of the following is not a type of orbital?
1. s
2. d
3. p
4. x
s
0%
d
0% 0%
p
0%
10
x
0
0
0
0
0
Team 1
Team 2
Team 3
Team 4
Team 5
Team Scores
An electron jumps up or down to a new energy level when
1.
the atom becomes charged.
2.
the atom becomes unstable.
3.
the electron’s location is pinpointed.
4.
the atom absorbs or emits energy.
0% 0% 0% 0%
th e at om
b ec om
...
th e at om
b ec om
...
th e el ec tro n’ s.
..
th e at om
a bs or
...
Which of the following statements is not true?
1.
An s orbital can hold two electrons.
2.
Each d orbital can hold up to two electrons.
3.
Each f orbital can hold up to three electrons.
4.
Each p orbital can hold two electrons.
0% 0% 0% 0%
A n s or d.
..
bi ta l c an
h ol
E ac h d or bi ta l c an
h o.
.
E ac h f o rb ita l c an
h ol
...
or bi ta l c an
h o.
.
E ac h p
A emission line spectrum is produced when an electron moves from one energy level
1. to a higher energy level.
2. to a lower energy level.
3. into the nucleus.
4. to another position in the same sublevel.
0% 0% 0% 0%
to
a
h ig he r ve l.
en er gy
le
to
a
lo w er
e ne rg y le ve l.
in to
th e nu cl eu s.
to
a no th er
p os iti on
i.
..
Because excited hydrogen atoms always produce the same line-emission spectrum, scientists concluded that hydrogen
1. had no electrons.
2. did not release photons.
3. released photons of only certain energies.
4. could only exist in the ground state.
0% 0% 0% 0%
h ad
n o el ec tr on s.
re
d id
n ot ph ot on s.
le as e
r el ea se d ph ot on s of
..
.
o nl y ex is t i n th
...
c ou ld
For an electron in an atom to change from the ground state to an excited state,
1.
energy must be released.
2.
energy must be absorbed.
3.
radiation must be emitted.
4.
the electron must make a transition from a higher to a lower energy level.
0% 0% 0% 0%
e ne rg y m us t b e re le a.
..
e ne rg y m us i..
.
t b e ab so
...
tio n m
r ad ia us t b e em
th e el ec tr on
m us t m a.
.
If electrons in an atom have the lowest possible energies, the atom is in the
1. ground state.
2. inert state.
3. excited state.
4. radiation-emitting state.
0% 0% 0% 0%
g ro un d st at e.
in er t s ta te
.
e xc ite d st at e.
r ad ia tio nem itt in g st at e.
1. s
2. p
3. d
4. f
Which orbital is this?
s
0%
p
0% 0%
d
0%
15
f
4580 Team 2
4481.6
7
Team 5
4453.3
3
Team 4
4250 Team 1
4178.3
3
Team 3
Team Scores
1. s
2. p
3. d
4. f
Which orbital is this?
s
0%
p
0% 0%
d
0%
10
f
1. B
If each of these sub orbitals contains 2 electrons, what element is this?
2. N
3. F
4. Ne
5. Na
B
0% 0%
N
0%
F
0%
N e
0%
N a
The electron in a hydrogen atom has its lowest total energy when the electron is in its
1. neutral state.
2. excited state.
3. ground state.
4. quantum state.
0% 0% 0% 0%
n eu tr al
s ta te
.
e xc ite d st at e.
g ro un d st at e.
q ua nt um
s ta te
.
The major difference between a
1 s orbital and a 2 s orbital is that
1.
the 2s orbital can hold more electrons.
2.
the 2s orbital has a slightly different shape.
3.
the 2s orbital is at a higher energy level.
4.
the 1s orbital can have only one electron.
0% 0% 0% 0%
th e
2s
o rb ita l c an
h o.
.
th e
2s
o rb ita l h
th e
2s
.
as
a
s l..
o rb ita l i s at
a
h
...
1s
o rb ita
th e l c an
h a.
.
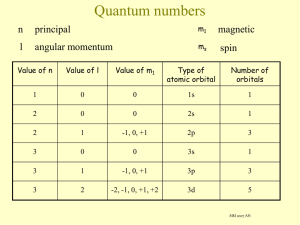
The letter designations for the first four sublevels with the maximum number of electrons that can be accommodated in each sublevel are
1. s :2, p :4, d :6, and f :8.
2. s :1, p :3, d :5, and f :7.
3. s :2, p :6, d :10, and f :14.
4. s :1, p :2, d :3, and f :4.
0% 0% 0% 0%
s
:2
, p
:4
, d
:6
, a nd
f:
8.
s
:1
, p
:3
, d
:5
, a nd
f:
7.
s
:2
, p
:6
, d
:1
0,
a nd
f:
14
.
s
:1
, p
:2
, d
:3
, a nd
f:
4.
Team Scores: 2 rounds left!!
5360 Team 2
5313.3
3
Team 4
5098.3
3
Team 5
4975 Team 1
4798.3
3
Team 3
The atomic sublevel with the next highest energy after 4 p is
1. 4 d .
2. 4 f .
3. 5 p .
4. 5 s .
4 d.
0% 0%
4 f.
0%
5 p.
0%
5 s.
The atomic sublevel with the next highest energy after 3s is
1. 3 d .
2. 2 f .
3. 3 p .
4. 4 s .
3 d.
0% 0%
2 f.
0%
3 p.
0%
4 s.
In the electron configuration for scandium
(atomic number 21), what is the notation for the three highest-energy electrons?
1. 4 s 2 3 d 1
2. 4 s 3
3. 3 d 3
4. 4 s 2 4 p 1
4 s2
3 d1
0% 0% 0% 0%
4 s3
3 d3
4 s2
4 p1
Which of the following lists atomic orbitals in the correct order they are filled according to the Aufbau principle?
1. 1 s 2 s 2 p 3 s 4 s 3 p 3 d 4 p 5 s
2. 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s
3. 1 s 2 s 2 p 3 s 3 p 4 s 4 p 3 d 4 d
4. 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 5 s
0% 0% 0% 0%
1 s
2s
2 p
3s
4 s
3p
3 d
..
1 s
2s
2 p
3s
3 p
1
4s
3 s
2s
2 d
..
4 p
..
p
3s
3 p
4s
1 s
2s
2 p
3s
3 p
3d
4 s
..
The element with electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 2 is
1. Mg ( Z = 12).
2. C ( Z = 6).
3. S ( Z = 16).
4. Si ( Z = 14).
0% 0% 0% 0%
M g
(Z
=
1
2)
.
C
(Z
=
6
).
S
(Z
=
1
6)
.
S i (
Z
=
14
).
4880 Team 2
4813.3
3
Team 4
4656.6
7
Team 5
4570 Team 1
4458.3
3
Team 3
Team Scores
What is the electron configuration for nitrogen, atomic number 7?
1. 1 s 2 2 s 2 2 p 3
2. 1 s 2 2 s 3 2 p 2
3. 1 s 2 2 s 3 2 p 1
4. 1 s 2 2 s 2 2 p 2 3 s 1
0% 0% 0% 0%
1 s2
2 s2
2 p3
1 s2
2 s3
2 p2
1 s2
2 s3
2 p1
1 s2
2 s2
2 p2
3 s1
The electron notation for aluminum (atomic number 13) is
1. 1 s 2 2 s 2 2 p 3 3 s 2 3 p 3 3 d 1 .
2. 1 s 2 2 s 2 2 p 6 3 s 2 2 d 1 .
3. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1 .
4. 1 s 2 2 s 2 2 p 9 .
0% 0% 0% 0%
1 s2
2 s2
2 p3
3 s2
3 p3
..
.
1 s2
2 s2
2 p6
3 s2 s2
2 s2
1
2 d1
.
2 p6
3 s2
3 p1
.
1 s2
2 s2
2 p9
.
If the s and p orbitals of the highest main energy level of an atom are filled with electrons, the atom has a(n)
1. electron pair.
2. octet.
3. empty d orbital.
4. electron in an excited state.
0% 0% 0% 0%
e le ct ro n pa ir.
o ct et
.
e m or bi ta l.
pt y d
e le ct ro n in
a n ex ci te d.
..
The number of electrons in the highest energy level of the argon atom (atomic number 18) is
1. 10.
2. 2.
3. 6.
4. 8.
10.
0% 0%
2.
0%
6.
0%
8.
If an element has an octet of electrons in its highest main energy level, there are ____ electrons in this level.
1. 2
2. 8
3. 10
4. 32
2
0%
8
0% 0% 0%
10 32
An element with 8 electrons in its highest main energy level is a(n)
1. octet element.
2. third period element.
3. Aufbau element.
4. noble gas.
0% 0% 0% 0%
o ct et
e le m en t.
th ird
p er io d el em en
A t.
uf ba u el em en t.
n ob le
g as
.
1900 Team 2
1806.6
7
Team 5
1800 Team 4
1765 Team 1
1673.3
3
Team 3
Team Scores
The three main groups of elements are metals, nonmetals, and
1. inert gases.
2. alkali metals.
3. radioactive isotopes.
4. metalloids.
0% 0% 0% 0%
in er t g as es
.
a lk al i m et al s.
r ad io ac tiv e is
...
m et al lo id s.
10
Most elements are
1. metals.
2. nonmetals.
3. metalloids.
4. semiconductors.
0% 0% 0% 0%
m et al s.
n on m et al s.
m et al lo id s.
s em ic on du ct or s.
10
Elements that do not conduct heat and electricity well are called insulators or ____.
1. metals.
2. nonmetals.
3. metalloids.
4. semiconductors.
0% 0% 0% 0%
m et al s.
n on m et al s.
m et al lo id s.
s em ic on du ct or s.
10
Alkaline-Earth metals are elements in group
1. 1
2. 2
3. 3-12
4. 17
1
0%
2
0% 0%
3
-1
2
0%
10
17
Halogens are elements in group
1. 1
2. 2
3. 3-12
4. 17
1
0%
2
0% 0%
3
-1
2
0%
10
17
Most elements on the right side of the periodic table are
1. semiconductors.
2. metals.
3. nonmetals.
4. metalloids.
0% 0% 0% 0%
s em ic on du ct or s.
m et al s.
n on m et al s.
m et al lo id s.
10
Team Scores with 10 to go
2400 Team 2
2306.6
7
Team 5
2165 Team 1
2153.3
3
Team 4
2093.3
3
Team 3
Which of the following is not true of noble gases?
1. They are highly reactive.
2. They exist as single atoms.
3. They belong to
Group 18.
4. They are nonmetals.
0% 0% 0% 0%
T he y ar e hi gh ly
T he
re ac tiv e.
y ex is t a
.
s si ng le
..
T he y be lo ng
to
G ro u.
..
T he y ar e no nm et al s.
Which statement about noble gases is correct?
1.
They form compounds with very bright colors.
2.
They exist as single atoms rather than as molecules.
3.
They are highly reactive with both metals and nonmetals.
4.
They are extremely rare in nature.
0% 0% 0% 0% po un
..
T he y fo rm
c om
T he y ex is
T
..
.
t a s si ng le he y ar e hi gh ly
re ac
T he y ar ti.
.
e ex tr em el y ra
..
Group 18 noble gases are relatively inert because
1.
they readily form positive ions.
2.
they can have either a positive or a negative charge.
3.
their outermost energy level is missing one electron.
4.
their s and p orbitals are filled.
0% 0% 0%
th ey
r ea di ly
fo rm
p os
...
th ey
c an
h av e ei th er
..
.
th ei r ou te rm os t e ne rg
...
an d p or bi ta ls
a
..
th ei r s
0%
Team Scores with 4 to go
3320 Team 2
3281.6
7
Team 5
3153.3
3
3093.3
3
Team 4
Team 3
3060 Team 1
Which is not a family of the periodic table?
1. alkaline-earth metals
2. Transition metals
3. halogens
4. noble gases
0% 0% 0% 0%
a lk al in eea rt h m et al s
T ra ns iti on
m et al s
h al og en s
n ob le
g as es
Elements that share properties of both metals and nonmetals are called
1. ions.
2. periods.
3. semiconductors.
4. valences.
0% 0% 0% 0%
io ns
.
p er io ds
.
s em ic on du ct or s.
v al en ce s.
The charge of an atom is
1. positive.
2. neutral.
3. negative.
4. unbalanced.
p os iti ve
.
0% 0% 0% 0%
n eu tr al
.
n eg at iv e.
u nb al an ce d.
The charge of a compound is
1. positive.
2. neutral.
3. negative.
4. unbalanced.
p os iti ve
.
0% 0% 0% 0%
n eu tr al
.
n eg at iv e.
u nb al an ce d.
Team Final Scores
5855 Team 2
5813.3
3
Team 4
Team 3 5603.3
3
5586.6
7
Team 1
5523.3
3
Team 5