Cooperative Binding
advertisement

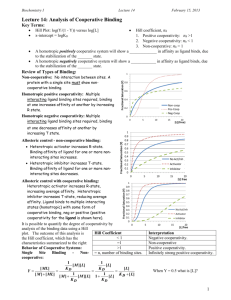
Cooperative Site Binding (11.8) • Binding of ligands to a biomolecule can affect the ability of other active sites to bind ligands and is called cooperative binding – Full cooperativity occurs when either all sites are occupied or unoccupied P NL PLN K CPLN CP CLN • In full cooperativity, the number of free sites is related to the number of free biopolymer molecules – Ratio of occupied to unoccupied sites is related to the ratio of bound biopolymer to free biopolymer CPLN CP N K C N N L • The Hill equation is used to determine the binding constant and the number of active sites on a biopolymer that exhibits full cooperativity – A normalized saturation parameter can be defined and is related to the fraction of active sites occupied on the biopolymer KCLN N 1 KCLN ln lnK N lnCL 1 Real World Cooperativity (11.8-11.9) • Fully cooperative binding is an idealized situation – Intermediate cooperativity can be described using the Hill parameter (α) – Hill parameter can range from 1 (independent binding) to N (fully cooperative), called positive cooperativity – Hill parameter can also be less than 1, called negative cooperativity KCL 1 KCL • Allosterism is the property of a protein where ligand binding regulates the activity of the protein – Structural changes in the protein as ligands bind are responsible for allosterism – Allosteric effects are often described by one of two models: concerted or sequential • Oxygen transport by hemoglobin and myoglobin are examples of cooperative and independent binding – Myoglobin exhibits independent binding (α=1 in a Hill plot) – Hemoglobin exhibits cooperative binding at intermediate partial pressures of oxygen – Hemoglobin exhibits independent binding at high and low oxygen concentrations Concerted and Sequential Models (11.9) • The concerted model describes ligand binding in a two-state model – Binding sites are either in a tense state (T) or a relaxed state (R), with the relaxed state having a higher binding affinity – All sites on the protein exist in either the T or R state – An equilibrium exists between the T and R forms, with the value of the equilibrium constant depending on the number of ligands bound to protein K0 CT CR • As the number of ligands bound to the protein increases, the equilibrium shifts to the relaxed form – High and low concentration binding of hemoglobin is explained well using the concerted model (binding becomes independent at high and low concentrations of oxygen) • Sequential model states that the binding of each active site is affected by the successive binding of ligands – A mix of tight and relaxed active sites is allowed – This model can be used to explain negative cooperativity Independent vs. Cooperative Binding Hemoglobin Myoglobin Binding in Hemoglobin and Myoglobin Hill Plot for Hemoglobin and Myoglobin Concerted Model Sequential Model