Study Guide Chapter 7 Protein Function 1. What is a ligand, a
advertisement

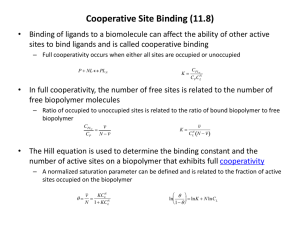
Study Guide Chapter 7 Protein Function 1. What is a ligand, a binding site, a substrate, a catalytic site, cooperativity? 2. What do we mean by the term induced fit? 3. Discuss how the protein myoglobin is designed to bind O2. What are the important features of this structure? 4. With respect to binding, what is 2? What does the binding curve of myoglobin look like? How can one find Kd (the dissociation constant) for the binding of O2 to myoglobin from this plot? 5. How is hemoglobin similar to and different than myoglobin? What does the binding curve of hemoglobin look like? Is it the same as or different than the binding curve of myoglobin. If it is different, why is it, and how is this important? 6. What are the T and R states of hemoglobin? How are they used to explain cooperativity in hemoglobin? 7. What is an allosteric protein, a homotropic modulator, a heterotropic modulator, a Hill plot, a Hill coefficeint? 8. What are the two major models used to explain cooperativty in hemoglobin? How are they different? 9. What is the Bohr effect? What is the importance of 2,3- biphophoglycerate? What is sickle cell anemia? What is the molecular defect that causes this disease, and what is the molecular explanation of how the disease works? Since this disease is associated with a fatal gene product, why haven’t all the people carrying this disease died, thus removing the disease from the gene pool?