pO 2
Protein Function
Myoglobin and Hemoglobin
O
2
Binding and Allosteric Properties of Hemoglobin
•
Hemoglobin binds and transports H
+
, O
CO
2
in an allosteric manner
2
and
•
Allosteric interaction –
of, relating to, undergoing, or being a change in the shape and activity of a protein (as an enzyme) that results from combination with another substance at a point other than the chemically active site
•
a regulatory mechanism where a small molecule(effector) binds and alters an enzymes activity
Protein Function
O
2 does not easily diffuse in muscle and O
2 is toxic to biological systems, so living systems have developed a way around this.
Physiological roles of:
– Myoglobin
Transports O
2 in rapidly respiring muscle
Monomer - single unit
Store of O
2 in muscle high affinity for O
2
Diving animals have large concentration of myoglobin to keep O
2 supplied to muscles
– Hemoglobin
Found in red blood cells
Carries O
2 from lungs to tissues and removes CO blood to lungs
2
Lower affinity for O
2 than myoglobin
Tetrameter - two sets of similar units (
2
2
) and H + from
– Made up of 8 helix A to H (proline near end)
– very small due to the folding
– hydrophobic residues oriented towards the interior of the protein
– only polar AAs inside are 2 histidines
– Red indicates
Heme group
x-ray crystallography of myoglobin
Structure of heme prosthetic group
Protoporphyrin ring w/ iron = heme
Four Pyrrole groups [A to D] linked by methane bridges
Fe +2 coordinated by prophyrin N atoms and a N from Histidine
(blue)
– This is known as His F8 (8 th residue of the F helix
Iron is out of plane due to His 8 bond
A molecule of O
2 ligand acts as 6 th
Structure of heme prosthetic group
Two hydrophobic sidechains on
O2 binding site of heme elp hold it in place
– Valine E11 and phenylalanine
CD1
oxygenation changes state of Fe
– Purple to red color of blood,
Fe +3 – brown
Oxidation of Fe +2 destroys biological activity of myoglobin
Physical barrier of protein is to maintain oxidation state of Fe +2
free vs. bound heme - role of apoprotein
Apoprotein the protein moiety of a molecule or complex
restricts heme dimers
keeps iron reduced
stabilizes transition state (O
2 binding)
CO, NO and H poison of O
2
2
S binding binding bind with greater affinity than O
2
His E 7 decreases affinity of ligands (CO and O
2
) for Fe +2
Myoglobin and Hemoglobin
Hemoglobin is structurally related to myoglobin very different primary sequence about an 18% homology in the primary sequence
2 alpha subunits and 2 beta subunits in adults there are very small amount of alpha
2 delta
2 hemoglobin
- significance of conserved amino acids between myoglobin and hemoglobin
• these are the important aas which keep hemes in contact with the protein
• Stabilizes helical arraignment
• Interacts with heme/iron
•
There are two general structural states - the deoxy or T form and the oxy or R form.
One type of interactions shift is the polar bonds between the alpha 1 and the beta 2 subunits.
3D structure of hemoglobin and myoglobin
1
1 units have 35 interactions
1
2 units have 19 interaction sites similar units have few polar contacts the two and two subunits face each other through aqueous channels
Binding of oxygen dramatically alters the interactions and brings about a twisting of the two halves (alpha beta pairs)
Much of the quaternary changes takes place in the salt bonds between the C terminals of all four chains
Myoglobin O
2
affinity
myoglobin -oxygen dissociation
MbO
2
<-> Mb + O
2
K =
[Mb] [O
2
]
[MbO
2
]
[MbO
2
]
Y =
[MbO
2
] + [O
2
]
[O
2
]
Y =
[O
2
] + K pO
2
Y = pO
2
+ P
50
Y = fraction of globin bound to O
2
K = dissociation constant
2
Myoglobin vs. Hemoglobin O
2 affinity
Note the hyperbolic vs sigmoidal nature of the two curves!
Sigmoidal curves indicate cooperativity
Hemoglobin -sigmoidal dissociation
Hb(O
2
) n
=Hb +n O
2
X n
= number of Hb subunits log
Y
= n log pO
2
n log P
50
Y = fraction of globin bound to O
2
1 - Y
n = Hill constant - determined graphically by the - hill plot n is the slope at midpoint of binding of log (Y/1-Y) vs log of pO
2 if n = 1 then non cooperativity if n < 1 then negative cooperativity if n >1 then positive cooperativity
•The experimentally determined slope does not reflect the number of binding sites however. It reflects, instead, the degree of cooperativity.
•
•
So how does this relate the biological effect of O affinity of Hb?
2 look at pO2 of alveoli vs. metabolically active tissue
What if oxygen dissociation were hyperbolic rather than sigmoidal?
Alteration in oxygen binding by:
- H+, CO
2
2,3 bisphosphoglycerate (BPG) effect of pH effect of BPG
% O
2
Sat % O
2
Sat pO
2 pO
2
Bohr Effect (CO
2
& pH effect on O
Simple rule of thumb-
2 binding)
– High H+ conc and higher CO
– High O
2
2 levels decrease O
2 concentration decrease H+ and CO
2 affinity for Hb affinity for Hb
Hb acts as a H+ buffer in respiring tissue
Cooperative Binding of O
2 to Hemoglobin
No cooperativity pH = 7.4
pH = 7.2
pH = 7.6
20 40 60 80 100 pO
2
(torr)
120
BPG and CO
2
Effects on O
2
Binding
Stripped Hb
Hb + BPG
Hb + BPG + CO
2
Hb + CO
2
20 40 60 80 100 pO
2
(torr)
120
Functional Structure of Hemoglobin allosteric regulations
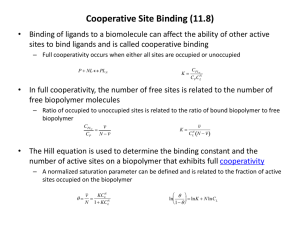
Allosteric interaction the binding of one ligand at one site in a protein that affects the binding of other ligands at other sites in the protein. This can be affect on binding can be cooperative (pos or neg).
Allosterism is typically seen when sigmoidal binding / activity curves.
Simple definition of the concept of cooperativity.
In its simplest meaning, cooperativity is a measure of communication between binding sites.
Positive cooperativity describes a relationship in which the binding to one site facilitates the binding to the second, third, ect.
No cooperativity puts all sites on equal footing and indicates no site to site influence. Negative cooperativity implies binding to one site hinders binding to subsequent sites.
So why is this important.
Look at the Hill plot and the plot of the of binding vs pO2 (figs 7-8 and 7-7 respectively). Think of when hemoglobin should be mostly saturated and when it would be best if it had a low saturation / affinity and thus "give up" its oxygen. Use the table below to help your thoughts.
Oxygen pressure in various fluids
Region or fluid pO
2
(Torr)
Inspired Air 158
Aveolar Air 100
Arterial Blood 90
Capilary
Insterstitual Fluid
40
30
Cell Cytosol 10
Look at the pO
2 pressure and think of where
Hb will be loaded with oxygen and where it will deliver oxygen
How is this cooperation actually forced on hemoglobin?
The structures in one subunit effects the others
• Structural differences of bound and unbound Hb
–
Two structures - T and R form depending on the oxygenation of Hb
–
–
T is the deoxy form, R is oxy form allosteric modifiers shift or stabilize one particular conformation over the other
– Monomer interactions, most chain interaction occurs between and chains
» through mostly hydrophobic residues
» and interactions are few and polar
» and contact act as a switch
oxy and deoxy quaternary structures are different
– change takes place between
1
– amino acids between forms
1 and
2
2 and
2
1 help to stabilize each
Oxygen binding shifts quaternary structure at long distances
–binding of O heme
2 ligand at 6th coordinate position pulls Fe into
–moves proximal histadine (F8) and the alpha helix it is attached to.
–shift in the helix is transmitted throughout of molecule
– The T form finds the terminals in several important H bonds and salt bridges.
– In the T form the C terminus of each subunit are "locked" into position through several hydrogen and ionic bonds.
– Shifts into the R state break these and allow an increased movement throughout the molecule.
Note that binding of one or more oxygen can have a dramatic affect on the other subunits that have not yet bound an O
2
.
How Do Myoglobin (Hemoglobin) Bind to Oxygen
Move down upon O binding
2
N
NH
His E7
How Do Myoglobin (Hemoglobin) Bind to Oxygen
Move down upon O binding
2
N
NH
His E7
T and R State of Hemoglobin - Review
Below are the two major conformations of hemoglobin as predicted by the models for allosteric activation.
Oxygen will bind to hemoglobin in either state; however, it has a signficantly higher affinity for hemoglobin in the R state.
In the absence of oxygen, hemoglobin is more stable in the T state, and is therefore the predominant form of deoxyhemoglobin. R stands for relaxed, while T stands for tense, since this is stabilized by a greater number of ion pairs.
Upon a conformational change from the T state to the R state, ion pairs are broken mainly between the
1
2 subunits.
Structural Changes when converting R to T Hb
Bottom Line:
CO
2
, BPG and H
+
all help to stabilize Hb in R or T form.
Bohr Effect Continued
The Bohr effect is the reversible shift in Hb affinity for O
2 changes in pH.
with
H+ Transport (effect) O
2 binding to Hb releases H + due to conformational changes in Hb
deoxyform (T form) brings Asp 94 close to His 146 (fig 7-11 (b))
- the proximity of an acidic amino acid increases the pK of histidine
(pKa is now above the pH) and results in H + “binding” to deoxyHb in other words the His becomes protonated where it normally would be ionized
increasing pH stimulates Hb to bind to O
-
Bottom line - when O
2
2 binds Hb, H+ is released from several amino acid's functional groups. When O
2 is released, the amino acids become protonized and then "picks" up a H+.
So when the H+ is high (acidic conditions) the H+ is driven onto the terminal amino acids driving it into the T conformation
CO
2 effects - In the red blood cells the picture is even more complicated. CO
2 is removed by converting CO
2 to bicarbonate
CO
2
+ H
2
O <-> HCO
3
+ H +
bicarbonate (HCO
3
) formed by the enzyme carbonic anhydrase
– H+ produced by this enzyme is removed by the Hb as described above
– This allows more CO
2 to be removed in the form of bicarbonate
CO
2 binding aids in reducing O more H+ (R to T change)
2 affinity by changing conformation by the production of
In the lungs the O the R conformation
2
binds Hb and forces
This all aids the function of Hb.
In active tissues respiration, (glycolysis) results in lactic acid formation.
These tissues need more O2. Without the H+ effect Hb would hold on to more of the O2. The H+ induces Hb to dump 10% more of it's O
–
2
.
CO
2 reversibly binds to N term (carbamate) to remove remaining CO
2
R - NH + CO
2
<-> R - NH - COO + H +
The carbamide increases the T formation - deoxy form.
The reverse occurs in the lungs. This results in 1/2 of CO2 removal from tissues.
H +
O
C
O H
H
2
N C C Protein
R O
Amino Terminus
H
O
C
H
N C C Protein
O
R O
Carbamate on Amino Terminus
–
2,3 bisphosphoglycerate
(BPG) Effects
Purified Hb has a different O
2 affinity than it does in blood
26 fold decrease change in affinity is due to 2,-3 diphosphoglycerate BPG
diphosphoglycerate BPG
present in human red blood cells at approximately 5 mmol/L.
It binds with greater affinity to deoxygenated hemoglobin
In bonding to partially deoxygenated hemoglobin it allosterically upregulates the release of the remaining oxygen molecules bound to the hemoglobin, thus enhancing the ability of RBCs to release oxygen near tissues that need it most
2,3 bisphosphoglycerate (BPG)
–
Purified Hb has a different O
2 affinity than it does in blood
26 fold decrease change in affinity is due to 2,-3 diphosphoglycerate BPG
– (BPG replaced by nucleotides
IHP and ATP in fish and birds)
– - 1 BPG per Hb - binds in central cavity of Hb
– - binds preferentially to deoxy
Hb
– - hydrophobic bonds with Lys and salt bridge with His
– - O
2 binding changes conformation and “kicks out” BPG
– change in altitude increases concentration of BPG
Fetal F Hb has replaced His 143 with Ser - What might the consequences be?
Cooperative interactions between subunits
Both models do not fully account for the effects of allosteric effectors
– sequential model (D Koshland)
binding of one O
2 induces T-R conformation change
1st change is most difficult due to influence by 3 other subunits
binding of next three subunits happens sequentially, with higher affinity (easier T-R changes) kinetics increase to the fully oxy Hb state as more O
2 is bound
Concerted model (J Monod)
All R or all T no in between as in the
Koshland model concerted model means as more O
2 binds, the R conformation is favored until all units are in the R conformation regardless of the total units bound to O
2
Affinities do NOT change until conformation changes
1 O
2
- all T; 2 O equilibrium; 3 O mostly R form
2
2
- nearly even mostly R; 4 O
2
energy from O
2 binding causes the change in equilibrium this model best fits O curve but with limits.
2 dissociation
Sickle-Cell Anemia, a Molecular Disease
One of the first “molecular” diseases found - sickle cell anemia
– sickle cell - blood cell is elongated , mis-shaped (sickle)
occurs at low O
2 concentration caused by hemoglobin aggregates
inflammation in capillaries and pain red blood cells break down - anemia
– between 10% of American blacks and 25% of African blacks are heterozygous for sickle cell anemia
– homozygous usually do not survive into adult hood
– heterozygous individuals usually have no problem except when in severe oxygen deprivation
Single amino acid (point mutation) HbS vs. HbA changes structure
– sickle cell b chains have a valine in place of glutamate
– leads to more Hb S (sickle cell) has 2 more + charges than normal hemoglobin
Glu -Val occurs on exterior of protein - does not change O
2 dissociation/allosteric properties of protein
Deoxy HbS precipitates
– oxyHb phenylalanine b85 and leucine b88 interior
–
– phe and leu shift to exterior
– create a sticky patch with valine (hydrophobic bonding) nucleation (cluster of aggregate) occurs logarithmically
– homozygous - 1000 times faster than heterozygous
– that means mixed genes can re-oxygenate faster than polymerization can occur
how can such a disease occur?
– highest concentration of gene mutation occurs where there is high incidence of malaria
– heterozygous individuals survive this disease better than those without
– malaria causing parasite lives in red blood cells during part of its life cycle
– partial sickling must interrupt life cycle of malaria parasite