3-MGD Lec 4 2014
advertisement

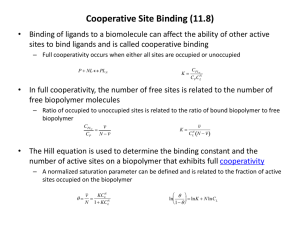
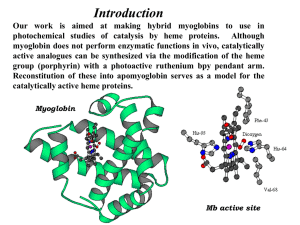
Molecules, Gene and disease Session 2 Lecture 4 Oxygen transport proteins Transport of O2 and CO2 by hemoglobin Haemoglobin and Myoglobin MYOGLOBIN • 153 amino acids (18 KDa) • O2-carrying protein in muscle • Contains essential prosthetic group (haem: contains Fe atom) • Compact protein, largely α-helices • Histidine involved in O2 –binding • Carbon monoxide can also bind Heme consists of: •an organic part, protoporphyrin, made up of 4 pyrrole rings,linked by methylene bridges (‘tetrapyrrole ring’) and with 4 methyl, 2 vinyl and 2 propionyl side chains; and •an atom of Fe, which binds to the N atoms of the protoporphyrin ring. 4 •The Fe can form 2 additional bonds, on either side of the plane. ferrous (+2) state binds the oxygen. one The Hemeprotein and Heme Features of myoglobin structure • Compact (45 x 35 x 25 Å) • 75% α-helical • Internal residues are non-polar, except for two His residues which are involved in O2-binding • The heme is largely hidden, but the propionate side-chains (-ve charge) are on the surface • Histidine F8 is directly linked to Fe • O2 is bound directly only to Fe in heme, on the opposite side to His F8 N Position of Fe in myoglobin. The Fe lies slightly out of the plane of the porphyrin ring, towards His F8. Oxygen binding to heme of myoglobin. O2-binding to Fe pulls the iron into the plane of the ring with associated movement of His F8. The binding of oxygen by myoglobin k1 Mb + O2 The equilibrium constant: Kd = MbO2 k-1 k-1 [Mb].[O2] 1 [MbO2] =k (1) Define the degree of saturation of myoglobin with oxygen (Y) as the concentration of oxymyoglobin as a proportion of the total myoglobin concentration: [MbO2] Y= (2) [Mb] + [MbO2] Substituting equation (1) into equation (2) gives: [O2] pO2 Y = K + [O ] = P + pO d 2 50 2 (As oxygen is a gas, its concentration is usually expressed in terms of partial pressure (pO2) and P50 is used to represent the equilibrium constant) This equation gives a hyperbolic relationship between Y and pO2, reminiscent of the MichaelisMenten equation (will be discussed in session 3). Binding of oxygen to myoglobin 1.0 0 0 pO2 5 (torr) 10 Haemoglobin • • 2 polypeptide chains: α (141 aa), β (146 aa) in α2β2 tetramer Oxygen-carrying protein in red blood cells • Each chain contains an essential heme prosthetic group • Conformation of each polypeptide chain is very similar to that of myoglobin • Sigmoidal O2-binding curve (cooperativity) Quaternary structure of Haemoglobin. The α2β2 tetramer contains 4 heme groups. Binding of Oxygen to hemoglobin Deoxy Oxy Transition from T to R state in haemoglobin On binding oxygen, one pair of αβ -subunits shifts with respect to the other by a rotation of 15 degrees. Binding of oxygen to one subunit ‘switches’ other subunits to a conformation which favours oxygen binding - leading to ‘cooperative’ binding of oxygen. Conformational changes in hemoglobin The movement of the iron atom on oxygenation brings the iron-associated His residue towards the porphyrin ring. The associated movement of the His-containing a helix alters the interface between the ab pairs and initiates other structural changes involving other subunits. Cooperative’ binding of oxygen to hemoglobin ‘Cooperative’ binding of oxygen to haemoglobin The binding curve can be pictured as a composite of two simple binding curves, of the kind seen for myoglobin - one corresponding to the T state (low affinity) and one to the R state (high affinity). As the concentration of substrate increases, the equilibrium shifts from the T state to the R state, resulting in a gradually increasing slope of the binding curve. Fractional saturation changes more steeply as a function of pO2. pO2 Allosteric effects • The ability o f hemoglobin t o reversibly bind oxygen is affected by the following parameters: • pH • pO2 • pCO2 • the availability o f 2,3-bisphosphoglycerate . These are collectively called allosteric ("other site") effectors , because their interaction at one site on the hemoglobin molecule affect s the binding of oxygen t o heme groups at other locations on the molecule . Regulation of oxygen binding The highly anionic 2,3-BPG is present in red blood cells at ~ 2 mM. It binds to haemoglobin (one molecule per tetramer) and decreases the affinity for O2, promoting release in the tissues. The physiological adaptation to high altitude involves increased tissue concentrations of BPG, leading to more efficient O2 release to compensate for the reduced O2 tension. Binding of 2,3-BPG to deoxyhaemoglobin 2,3-BPG binds in the central cavity of the tetramer, interacting with three positively charged groups (2 His, 1 Lys) on each b chain. The oxygenated haemoglobin has a smaller central gap and excludes 2,3-BPG. Differential oxygen affinity of foetal and maternal red blood cells Foetal haemoglobin contains a variant of the β chain, called ɤ, which has a HisSer substitution in the 2,3-BPG-binding site. The foetal haemoglobin thus has a reduced affinity for 2,3-BPG, resulting in an enhanced O2-binding affinity that allows transfer of O2 from the maternal to the foetal red blood cells The Bohr effect: H+ ions and CO2 promote the release of O2 Rapidly metabolising tissues, such as contracting muscle, have a high need for O2 and generate large amounts of H+ and CO2. Both of these species interact with haemoglobin to promote O2-release. The O2 affinity decreases as the pH decreases from the pH 7.4 found in the lungs. Increased CO2 concentrations also lead to a decrease in O2-affinity. This regulation of O2-affinity by pH and CO2 is called the Bohr effect after its discoverer, Christian Bohr (1904). Chemical Basis of the Bohr effect In deoxyhaemoglobin, three amino acid residues form two salt bridges that stabilise the T state (the low oxygen affinity state). The formation of one of these depends on the presence of a proton on His b146. Lowering of the pH by metabolic activity favours the proton-addition and formation of the salt bridge with Aspb94, thus stabilising the T structure and increasing the tendency for O2 to be released. The effect of CO2 on deoxyhaemoglobin The CO2 reacts with the terminal aNH2 groups to form carbamates, which are negatively charged. The N-termini lie at the interface between the ab dimers, and the negatively charged carbamates participate in salt-bridge interactions that favour the T state, stabilising the deoxy-form and favouring the release of O2 Carbon monoxide and haem iron Carbon monoxide is a poison because it combines with ferromyoglobin and ferrohaemoglobin and blocks oxygen transport. Note that HisE7 sterically inhibits binding of CO, and lowers its affinity for the haem Fe. This is then sufficiently low that endogenous levels of CO can be tolerated. High levels of CO from poorly ventilated gas fires are highly toxic. HisF8 N-Fe-O (Plane of porphyrin ring) O HisF8 N-Fe-C (Plane of porphyrin ring) O Summary