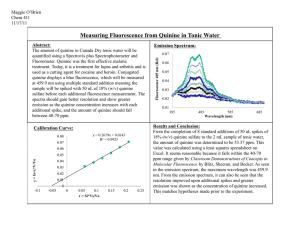
Fluorescence decay of QS
advertisement

CH332 Lab 4: FLUORESCENCE LIFETIME OF QUININE SULFATE Pre-lab: This exercise requires a 2.00 x 10-5 M quinine sulfate dihydrate solution in 0.5 M H2SO4 (trace metal grade only/ePure water only) and two different samples of the quinine solution with halide Cl(NaCl) quenching in the range of 0 to 8 x 10-2 M. Please show your calculations for the preparation of 250 mL of the quinine stock solution and 50 mL of two (different) quenched samples. In this experiment you will be concerned with the decay kinetics of the fluorescence of quinine sulfate in water solutions and the physical processes that effect the lifetime of QS. Quinine is used as a fluorescence standard because the fluorescence is not quenched (decreased) by oxygen but can be quenched in a controlled manner by adding atomic anions (F-, Cl-, I- and Br-) to the solution. Background Reading: Gutow, Jonathan H. J. Halide (Cl-) Quenching of Quinine Sulfate Fluorescence: A Time-Resolved Fluorescence Experiment for Physical ChemistryChem. Educ. 2005 82 302.) Instrument Details: Please note that we will be using the Quanta Master Instrument from PTI with a pulsed nano-LED as an excitation source instead of a laser. The optical diagram of our instrument is shown here. Your lab instructor will show you how to turn on the instrument. The instrument is controlled using Felix32 software running on the Acer PC. Details on each acquisition technique are provided below. You should first take an emission scan of quinine sulfate and then measure the fluorescence decay of three solutions (quinine sulfate and two solutions with different halide concentrations). Record your halide concentrations and decay rates in the table below. Each lab group will use the pooled decay data to complete the quenching plots and determine the quenching rate constant. 1) Take an absorbance spectrum of your unquenched and quenched quinine sulfate samples. Use the Ocean Optics Spectrometer. 2) Measure the emission spectra of Quinine Sulfate. Excitation or Emission Scan Tutorial, Text Directions (scroll to Emission). 3) Measure the fluorescence decay of a scattering solution (scattering reference) and your samples. The scattering sample is a dilute solution of coffee creamer. Timebase. You will find it helpful to read about Time Resolved Basics. Data analysis is described on page 144 of the manual and online (Decay Rate Calculations). Group [NaCl] Lifetime (nsec) ( [Cl- quenched sample] ) (actual [Cl-]) blank 0 blank 0 1 X 10-2 1 X 10-2 2 X 10-2 2 X 10-2 3 X 10-2 3 X 10-2 4 X 10-2 4 X 10-2 5 X 10-2 5 X 10-2 6 X 10-2 6 X 10-2 7 X 10-2 7 X 10-2 8 X 10-2 8 X 10-2 Questions: 1. What is the value of kq? What does kq refer to? Is the quenching of quinine sulfate static or dynamic? Explain. Are you confident in your data? (include stats!) 2. Compare and contrast the two fluorescence methods used in this lab (scans vs lifetime). Be specific regarding the information obtained in each experiment and differences in instrument operation. 3. How could this instrument be used to analyze two different compounds with similar emission and excitation spectra but very different fluorescence lifetimes? 4. Why is it possible to use a 310 nm pulsed diode to excite the sample when the maximum excitation wavelength is 350 nm? 5. How would the results of this experiment change if you ran the entire experiment at 5 degrees C? 6. What do the absorbance spectra of the unquenched and quenched quinine samples show (in terms of [Cl-])? Why is this an important piece of information? (Based on the article: Gutow, Jonathan H. J. Halide (Cl-) Quenching of Quinine Sulfate Fluorescence: A Time-Resolved Fluorescence Experiment for Physical ChemistryChem. Educ. 2005 82 302.)