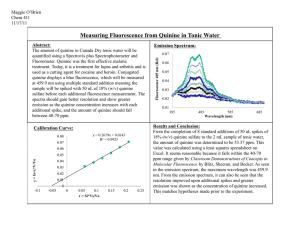
Quinine in Soft Drinks by Fluorimetry F2
advertisement

F2 Quinine in Soft Drinks by Fluorimetry Last Revised: March 2015 1. REAGENTS & EQUIPMENT 1.1 50 mg/L quinine in 0.1 M H2SO4 1.2 0.1 M H2SO4 1.3 Tonic water 1.4 Bitter lemon 2. PROCEDURE 2A. Prework Typical levels for quinine in bitter lemon and tonic water are 30 and 60 mg/L, respectively. Determine suitable dilutions in 100 mL. See Step 2.1 for the standard concentrations. 2B. Solution preparation 2.1 From the 50 mg/L standard, prepare 100 mL of 1, 5 and 10 mg/L calibration standards. You must use 0.1 M H2SO4 as the solvent in all solutions. 2.2 Prepare the sample dilutions in duplicate as determined in Prework 1. 2.3 Prepare duplicate recovery checks by pipetting 5 mL of the 50 mg/L standard into 100 mL volumetric flasks, together with the same volume of bitter lemon as in 2.2. 2C. Analysis 2.4 Record the UV/VIS spectrum of the 10 mg/L standard from 300-400 nm. Record the wavelength and absorbance for the peak. 2.5 Determine the appropriate filters for the analysis. 2.6 Zero the instrument with 0.1 M H2SO4 and adjust the gain to at least 1000 on an appropriate scale using the 10 mg/L standard. Check the zero again. Do not touch the Gain scale controls again. 2.7 Record the intensities of all solutions. 2.8 For the two samples, dilute roughly 1:1 in the sample tube and re-measure. 3. REPORT Calculations • plot a calibration graph of intensity vs concentration for the standards • determine the analyte concentration for each diluted sample • apply the dilution factor to determine the concentration in the original samples • calculate the % recovery as below, where the concentrations for the recovery check (RC) and bitter lemon (BL) solutions are those directly from the calibration graph %RC 100x AvgeRCconc AvgeBLconc 2.5 Discussion • compare your results to the expected values • explain why it is necessary to do a ‘rough’ 1:1 dilution on the sample even when its intensity is within the standards • compare the technique to UV/VIS absorption spectrophotometry for this analysis • if you have completed V3, compare the two techniques for the analysis of quinine (if you have already made this comparison in your V3 report, you don’t need to do it again) Questions 1. What is the structure of quinine? Explain why it is fluorescent. 2. What is the natural source of quinine? What was its major use for many years? 3. What is the meaning of the symbols SC and NB on the filters with this instrument? 4. List four matrix factors that can cause quenching of the fluorescent intensity. 5. Chemiluminescence is a recent analytical development, related to fluorescence. Explain how it occurs, and describe one important analytical application in the area of air pollution monitoring.. F2 p2 F2. QUININE IN SOFT DRINKS RESULTS SHEET Maximum absorbance Wavelength at max. A 1st filter 2nd filter Tonic water dilution factor Bitter lemon dilution factor Recovery check amount Solution 1 mg/L 5 mg/L 10 mg/L BL1 BL2 TW1 TW2 RC1 RC2 Intensity Gain