InorgCh11.1

A.
Electronic Spectra (Chapter 11 pp. 380-381)
1) A characteristic of transition metal complexes is color arising from electronic transitions between d-orbitals of different energies a) Everyday Examples of colored transition metal complexes
“Both rubies and sapphires owe their intense colors to impurities, ruby to the presence of chromium, and blue sapphire to both titanium and iron. In rubies, the color can be explained by crystal field theory, but in sapphire, a slightly different process, known as charge transfer, produces the color blue.” http://www.webexhibits.org/causes ofcolor/8.html b) The UV-Vis Experiment and the spectral result
2) The Beer-Lambert Law a) Absorbance = A = log (I o
/I) Most modern systems give readings in A directly, so you don’t have to do the math. A has no unit.
b) A = e l c describes the absorption of light in a solution i.
l
= the length of the cell containing the solution, usual 1 cm ii.
c = concentration in mol/L = M iii.
e
= Molar extinction coefficient = constant for a given molecule at a given wavelength of light = how well the molecule absorbs light
ε
A l c
1
(cm)(M)
M
1 cm
1
3) Plotting Spectra a) E = h n c = ln
= 3 x 10 8 m/s h = 6.626 x 10 -34 Js = Planck’s constant
E
hc
energy of light of wavelengt h
λ
λ
ν
1
λ
cm
1 b) Wavenumber = i.
higher energy = larger wavenumbers ii.
Higher energy = smaller wavelength
500 nm
??
cm
1 ν
1
(500nm)
10
7 cm nm
20 , 000 cm
1
E
hc
λ
hc ν
4) Color a) What we see as the color of a compound is the complementary color to what the compound absorbs b) Example: Absorbs red, we see green c) Example: Absorbs yellow/orange, we see blue/purple d) Many complexes have multiple absorption band, so prediction is hard
B.
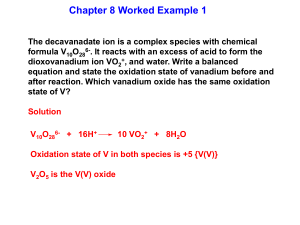
Interpreting the Electronic Spectrum of d 1 Ti 3+
1.
Single d-electron species are relatively easy to understand
2.
D o can be calculated directly from the energy of light absorbed
D o
1
493 nm
1 nm
1 x 10
7 cm
20 , 300 cm
1
C.
Interpreting the Spectra of Multi-Electron Species
1.
Electron-Electron Interactions complicate the spectra of multi-electron species
2.
Example: [V(H
2
O)
6
] 3+ is an octahedral d 2 metal ion
[V(H
2
O)
6
] 3+
286nm 333nm 400nm 500nm 667nm
3.
Because of the multiple electrons, now we have multiple excited states a) d xy
1 d xz
1 is not the only possible ground state, but is convenient to use b) We can promote one e- from d xy or d xz to d z2 or d x2-y2
(we show 2 above) c) Excited states produced: d xy
1 d z2
1 and d xy
1 d x2-y2
1 d) The energy needed for this excitation is not the same for each:
D
E a
≠ D
E b d xy
1 d x2-y2
1 will be higher energy because more repulsion; both in xy plane
e) Excitation d xy
1 d xz
1 ----> d xy
2 =
D
E c i.
Energy change is for pairing the electrons ii.
Absorption is extremely week (“spin forbidden” to spin flip)
4.
Example: [Mn(H
2
O)
6
] 2+ a) This spectrum has many very weak; solution is very pale
[Mn(H
2
O)
6
] 2+ b) Weak field ligand makes this a high spin octahedral d 5 metal ion c) All possible transitions include a “spin forbidden” spin flip, so all absorptions are very weak
5.
Example: [Cr(NH
3
)
6
] 3+ a.
There are two absorptions in its visible spectrum, one strong and one weak b.
Explanation i.
Octahedral d 3 metal ion has two types of excitations available ii.
One is “allowed”, the other spin forbidden iii.
Relatively strong field NH
3 makes
D o
(450nm) > P (650nm)
6.
The Spectrochemical Series and Electronic Spectra of Metal Complexes a) Ligand field strength affects
D o
, which effects the l of light absorbed b) It is difficult to correlate color with ligand field strength due to multiple absorbances of the multi-electron metal ions c) A particular metal ion’s color may correlate with related ligands
NO
2
-
Cl-
I-
d) Example: A sample of one Cr 3+ complex is green and another is yellow.
One is [Cr(NH
3
)
6
] 3+ and the other is [CrF
6
] 3. Which is which?
i.
Determine the color of light absorbed to determine energy ii.
Green complex absorbs red (less energetic) iii.
Yellow complex absorbs blue (more energetic) iv.
NH
3 complex must be yellow and F complex must be green e) Example: Assign the following two spectra to [Ni(H
2
O)
6
] 2+ and [Ni(NH
3
)
6
] 2+ and determine the color of their solutions.
i.
NH
3 is stronger field, so it should have higher energy peaks = (a) ii.
The NH
3 complex absorbs all but ~750nm, so solution is red iii.
The H
2
O complex absorbs all but ~500nm, so solution is green = (b) f) Example: A sample of one Co 3+ complex is green and another is yellow.
One is [Co(F)
6
] 3and the other is [Cr(CN)
6
] 3. Which is which?
i.
Green complex absorbs red (low energy) = F- complex (weak field) ii.
Yellow complex absorbs violet (high energy) = CN- complex (strong)