Chapt24
advertisement

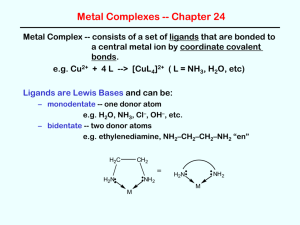
Metal Complexes -- Chapter 24 (Sections 24.3 - 24.5) 1. Metal Complex -- consists of a set of ligands that are bonded to a central metal coordinate covalent bonds. e.g., Cu2+ + 4 L ion CuL42+ (L = NH3, H2O, etc.) (a) Ligands are Lewis Bases and can be: monodentate -- bidentate -- two donor atoms one donor atom e.g., H2O, NH3, Cl-, OH-, etc. e.g., ethylenediamine, NH2-CH2-CH2-NH2 "en" H2C H2C H2N: : NH2 = H2N: : NH2 M M polydentate -- more than two donor atoms e.g., EDTA -- ethylenediaminetetraacetic acid (6 donor atoms) O .. :O .. C CH2 :N .. :O .. C CH2 O O CH2 CH2 .. : H2C C O .. N: .. H2C C O : .. O 1 = EDTA4- by (b) Writing Formulas of complex ions metal ion first, then ligands total charge = sum of metal ion + ligands e.g., metal ion ligand complex Cu2+ H2O Cu(H2O)42+ Co3+ NH3 Co(NH3)63+ Fe3+ CN- Fe(CN)63- (c) Chelate Effect - complexes with bi- or polydentate ligands are more stable than those with similar monodentate ligands e.g., Ni(en)33+ is more stable than Ni(NH3)63+ 3+ N N N N N Ni N (d) Nomenclature -- study rules and examples in book! Complex Ni(CN)42- Name tetracyanonickelate(II) ion CoCl63- hexachlorocobaltate(III) ion Co(NH3)4Cl2+ Na3[Co(NO2)6] tetraamminedichlorocobalt(III) ion [Cr(en)2Cl2]2SO4 dichlorobis(ethylenediamine)chromium(III) sulfate sodium hexanitrocobaltate(III) 2 2. Coordination Number and Structure Coord # = number of donor atoms attached to the metal center (a) Two-Coordinate Complexes -- linear structures rare except for Ag+ e.g., Ag(NH3)2+ and Ag(CN)2(b) Four-Coordinate Complexes -- two structural types Tetrahedral structures -- common for ions with filled d subshells, e.g., Zn2+ as in Zn(OH)42- 2- OH Zn OH HO OH Square Planar structures -- common for d8 metal ions (Ni2+, Pd2+, Pt2+) and for Cu2+ e.g., Cl Cl 2- H3N 2+ Cu Pt Cl NH3 H3N Cl NH3 (c) Six-Coordinate Complexes -- the most common! "always" octahedral structures, e.g., 3+ N N N Ni Cl N N H3N H3N N Cr Cl 3 NH3 Cl 3. Isomers of Coordination Complexes Isomers -- same chemical composition (formula) but different structures (due to either the arrangement of atoms or 3-D shape) (a) Linkage isomers same ligand, with different donor atoms e.g., In [Co(NH3)5NO2]2+, the NO2- ligand can bind to Co through N ("nitro") or O ("nitrito"). H3N NH 3 O H3N Co N O H3N NH 3 2+ 2+ H 3N H 3N Co H3N nitro complex NH3 O N O NH3 nitrito complex 4 (b) Geometrical isomers Square Planar complexes Cl NH3 Cl NH3 Pt Pt Cl NH3 H3N cis Cl trans "cisplatin" (anticancer drug) (no biological activity) Octahedral complexes Cl H3N H3N Cr Cl NH3 Cl H3N H3N NH3 NH3 NH3 Cr Cl cis trans (c) Enantiomers -- non-superimposable mirror images also called "optical isomers" N N N Co N N Cl N Cl Cl Co Cl 5 N N 4. Crystal Field Theory (Bonding in Transition Metal Complexes) Metal complexes are usually highly colored and are often paramagnetic – such facts can be explained by a "d-orbital splitting diagram" dz2 dx2-y2 eg = crystal field energy splitting energy dxy dxz dyz t2g d orbitals of the metal ion in an octahedral field of ligands d orbitals of the free metal ion The size of depends on the nature of the ligand "spectrochemical series" -- decreases: CN- > NO2- > en > NH3 > H2O > OH- > F- > Cl- > Br"strong field ligands" "weak field ligands" the oxidation state of the metal is greater for M3+ than for M2+ the row of the metal in the periodic table for a given ligand and oxidation state of the metal, increases going down in a group e.g., is greater in Ru(NH3)63+ than in Fe(NH3)63+ Colors of metal complexes are due to electronic transitions between the t2g and eg energy levels 6 d orbital splitting diagrams for octahedral complexes dz2 dz2 dxy dx2-y2 dxz dx2-y2 eg large "low spin" eg small "high spin" dyz t2g dxy Fe(H2O)62+ dxz dyz t2g Fe(CN)64- CN- is a stronger field ligand than is H2O which leads to a greater value (i.e., a greater d orbital splitting) as a result, Fe(H2O)62+ is a "high spin" complex and is paramagnetic (4 unpaired electrons) while, Fe(CN)64- is a "low spin" complex and is diamagnetic (no unpaired electrons) the CN- complex with the larger value absorbs light of higher energy (i.e., higher frequency but shorter wavelength) OMIT: d orbital splitting diagrams for other geometries (i.e., tetrahedral and square planar) 7