File
Transition Metal Chemistry and
Coordination Compounds
Lecture 18-20
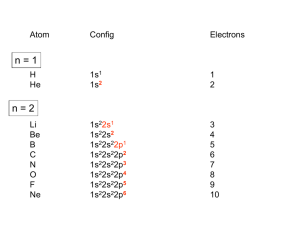
The Transition Metals
Oxidation States of the 1 st Row Transition Metals
( most stable oxidation numbers are shown in red )
Mn(II)
Aqueous oxoanions of transition elements
One of the most characteristic chemical properties of these elements is the occurrence of multiple oxidation states.
Mn(VI) Mn(VII)
V(V)
Cr(VI)
Mn(VII)
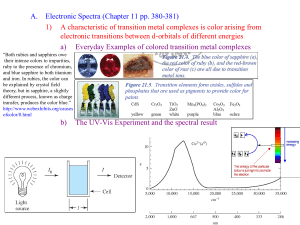
Effects of the metal oxidation state and of ligand identity on color
[V(H
2
O)
6
] 2+
[V(H
2
O)
6
] 3+
[Cr(NH
3
)
6
] 3+ [Cr(NH
3
)
5
Cl ] 2+
Ionization Energies for the 1 st Row Transition Metals
Scandium Titanium
Chromium Manganese
Vanadium
Iron
Cobalt Nickel Copper
Coordination Compounds
A coordination compound typically consists of a complex ion and a counter ion.
A complex ion contains a central metal cation bonded to one or more molecules or ions.
The molecules or ions that surround the metal in a complex ion are called ligands .
A ligand has at least one unshared pair of valence electrons
H
O
H
• •
N
H
H
H
• •
••Cl
-
C O
22.3
Coordination Compounds
The atom in a ligand that is bound directly to the metal atom is the donor atom .
O
• •
N
H H H
H
H
The number of donor atoms surrounding the central metal atom in a complex ion is the coordination number .
Ligands with: one donor atom two donor atoms monodentate bidentate three or more donor atoms polydentate
H
2
O, NH
3
, Cl ethylenediamine
EDTA
Coordination Compounds bidentate ligand
H
2
• •
N CH
2
CH
2
• •
NH
2 polydentate ligand
(EDTA)
Bidentate and polydentate ligands are called chelating agents
EDTA Complex of Lead
What are the oxidation numbers of the metals in
K[Au(OH)
4
] and [Cr(NH
3
)
6
](NO
3
)
3
?
OH has charge of -1
K + has charge of +1
? Au + 1 + 4x(-1) = 0
Au = +3
NO
3
has charge of -1
NH
3 has no charge
? Cr + 6x(0) + 3x(-1) = 0
Cr = +3
Naming Coordination Compounds
• The cation is named before the anion.
• Within a complex ion, the ligands are named first in alphabetical order and the metal atom is named last.
• The names of anionic ligands end with the letter o . Neutral ligands are usually called by the name of the molecule. The exceptions are H
2
O (aqua), CO (carbonyl), and NH
3
(ammine).
• When several ligands of a particular kind are present, the
Greek prefixes di, tri, tetra, penta -, and hexaare used to indicate the number. If the ligand contains a Greek prefix, use the prefixes bis , tris , and tetrakis to indicate the number.
• The oxidation number of the metal is written in Roman numerals following the name of the metal.
• If the complex is an anion, its name ends in –ate .
22.3
What is the systematic name of
[Cr(H
2
O)
4
Cl
2
]Cl ?
tetraaquodichlorochromium(III) chloride
Write the formula of tris(ethylenediamine)cobalt(II) sulfate
[Co(en)
3
]SO
4
Crystal-Field Theory
• Model explaining bonding for transition metal complexes
– • Originally developed to explain properties for crystalline material
– • Basic idea:
–
Electrostatic interaction between lone-pair electrons result in coordination.
Energetics
• CFT - Electrostatic between metal ion and donor atom
i i) Separate metal and ligand high energy ii) Coordinated Metal - ligand stabilized iii) Destabilization due to ligand -d electron repulsion iv) Splitting due to octahedral field.
ii iii iv
d-Orbitals and Ligand
Interaction
(Octahedral Field)
•Ligand s approac h metal
d-orbitals pointing directly at axis are affected most by electrostatic interaction d-orbitals not pointing directly at axis are least affected
(stabilized) by electrostatic interaction
Ligand-Metal Interaction
•Crystal Field Theory - Describes bonding in Metal Complexes
• Basic Assumption in CFT:
• Electrostatic interaction between ligand and metal
d-orbitals align along the octahedral axis will be affected the most.
More directly the ligand attacks the metal orbital, the higher the energy of the d-orbital.
In an octahedral field the degeneracy of the five d-orbitals is lifted
Splitting of the d-Orbitals
• Octahedral field Splitting Pattern:
•
The energy gap is referred to as
(10 Dq) , the crystal field splitting energy.
The d z
2 and d x
2 y
2 orbitals lie on the same axes as negative charges.
Therefore, there is a large, unfavorable interaction between ligand (-) orbitals.
These orbitals form the degenerate high energy pair of energy levels.
The d xy
, d yx and d xz orbitals bisect the negative charges.
Therefore, there is a smaller repulsion between ligand & metal for these orbitals.
These orbitals form the degenerate low energy set of energy levels.
Splitting of d orbitals in an octahedral field
3/5 o e g
2/5 o t
2g
o is the crystal field splitting
E( t
2g
) = -0.4
o x 3 = -1.2
o
E( e g
) = +0.6
o x 2 = +1.2
o
o
The magnitude of the splitting
(ligand effect)
Strong field
Weak field
The spectrochemical series
CO, CN > phen > NO
2
> en > NH
3
> NCS > H
2
O > F > RCO
2
> OH
-
> Cl > Br > I -
The magnitude of the splitting
(metal ion effect)
Strong field
Weak field
increases with increasing formal charge on the metal ion
increases on going down the periodic table
The spectrochemical series
•For a given ligand, the color depends on the oxidation state of the metal ion.
•For a given metal ion, the color depends on the ligand.
I < Cl < F < OH < H
2
O < SCN < NH
3
< en < NO
2
< CN < CO
WEAKER FIELD STRONGER FIELD
SMALLER
LONGER
LARGER
SHORTER
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Electron Configuration in Octahedral
Field
• Electron configuration of metal ion:
• s-electrons are lost first.
• Ti 3+ is a d
• Hund's rule:
1 , V 3+ is d 2 , and Cr 3+ is d 3
• First three electrons are in separate d orbitals with their spins parallel.
• Fourth e- has choice:
• Higher orbital if is small; High spin
• Lower orbital if is large: Low spin.
• Weak field ligands
• Small , High spin complex
• Strong field Ligands
• Large , Low spin complex
d 1
Placing electrons in d orbitals
Strong field Weak field
Strong field Weak field d 2 d 3 d 4
When the 4 th electron is assigned it will either go into the higher energy e g orbital at an energy cost of cost of P , the pairing energy.
0 or be paired at an energy d 4
Strong field =
Low spin
(2 unpaired)
Weak field =
High spin
(4 unpaired)
P
<
o
P
>
o
Pairing Energy,
P
The pairing energy, P , is made up of two parts.
1) Coulombic repulsion energy caused by having two electrons in same orbital. Destabilizing energy contribution of P c for each doubly occupied orbital.
2) Exchange stabilizing energy for each pair of electrons having the same spin and same energy. Stabilizing contribution of P e for each pair having same spin and same energy
P = sum of all P c and P e interactions
Placing electrons in d orbitals d 5 d 6 d 7
1 u.e.
5 u.e.
d 8
0 u.e.
4 u.e.
d 9
1 u.e.
3 u.e.
d 10
2 u.e.
2 u.e.
1 u.e.
1 u.e.
0 u.e.
0 u.e.
To sum up
• Electron Configuration for Octahedral complexes of metal ion having d 1 to d 10 configuration [M(H
2
O)
6
] +n .
• Only the d 4 through d 7 cases have both high-spin and low spin configuration .
Electron configurations for octahedral complexes of metal ions having from d 1 to d 10 configurations. Only the d 4 through d 7 cases have both high-spin and low-spin configurations.
Colour in Coordination Compounds
E = h n
22.5
The absorption maximum for the complex ion
[Co(NH
3
)
6
] 3+ occurs at 470 nm. What is the color of the complex and what is the crystal field splitting in kJ/mol?
Absorbs blue, will appear orange.
= h n
= hc
=
(6.63 x 10 -34 J s) x (3.00 x 10 8 m s -1 )
470 x 10 -9 m
= 4.23 x 10 -19 J
(kJ/mol) = 4.23 x 10 -19 J/atom x 6.022 x 10 23 atoms/mol
= 255 kJ/mol
22.5
Color Absorption of Co
3+
Complexes
• The Colors of Some Complexes
[CoF
6
]
of the Co
3+
Ion
Red Green
600, 420 Yellow, violet Dark green [Co(C
2
O
4
)
3
] 3+
[Co(H
2
O)
6
] 3+
[Co(NH
3
)
6
] 3+
[Co(en)
3
] 3+
[Co(CN)
6
] 3+
600, 400
475, 340
470, 340
310
Yellow, violet
Blue, violet
Blue, ultraviolet Yellow-orange
Ultraviolet
Blue-green
Yellow-orange
Pale Yellow
The complex with fluoride ion, [CoF
6
] 3+ , is high spin and has one absorption band.
The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band.
Colors & How We
Perceive it
650
800
Artist color wheel showing the colors which are complementary to one another and the wavelength range of each color.
400
430
580
560
490
Complex Influence on Color
•Compounds of Transition metal complexes solution.
[Fe(H
2
O)
6
] 3+
[Co(H
2
O)
6
[Ni(H
] 2+
2
O)
6
] 2+
[Cu(H
2
O)
[Zn(H
6
] 2+
2
O)
6
] 2+
800
400
650
430
580
560
490