Complex Ions and Free Energy
advertisement

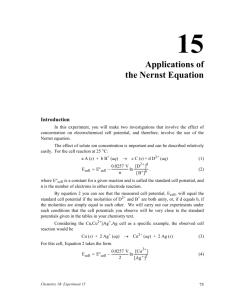
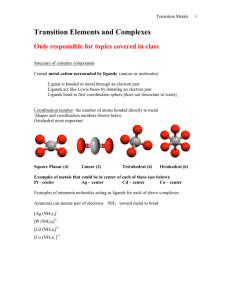
Catalyst 1. Calculate the Ksp for AgC2H3O2 whose mass solubility is 1.02 g/100 mL 2. A solution containing 0.010 M Ba2+ and 0.10 M Ag+, which solid will precipitate first when Na2SO4 is added to the solution? Justify your reasoning with calculations. NOTE the Ksp (BaSO4) = 1.1 x 10-10 and Ksp (AgSO4) = 1.1 x 10-5. End Cobalt Complexes Lecture 8.3 – Complex Ion Formation, Equilibrium Constants, and ΔG Today’s Learning Targets • LT 8.6 – I can discuss how coordination complexes form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly soluble compound. • LT 8.8 – I can calculate the free energy of a chemical reaction by utilizing my knowledge the value of K. Transition Metal Complexes • When transition metals bond they are the central atom and they bond extensively with numerous substances. • The complexes that form are known as metal complexes • The molecule that bond with metals are known as ligands ▫ A ligand is always a Lewis Base • The number of ligands bound to the metal is the coordination number Common Ligands • The most common ligands are H2O, NH3, Cl-, and CN- due to their free lone pair of electrons that can be donated to a metal. The Metal Ligand Bond • The bond between a metal and a ligand is a Lewis acid-base interaction • Metal = electron pair acceptor (acid) • Ligand = electron pair donor (base) Formation of Complex Ions • The formation of complex ions can drastically impact the solubility of a metal. • Ligands act to pull metal ions into solution and displace solvating H2O, thus, making the solution more soluble AgCl (s) Ag+ (aq) + Cl- (aq) Ag+ (aq) + 2NH+3 (aq) Ag(NH3 ) 2 (aq) Overall: AgCl (s)+ 2NH+3 (aq) Ag(NH3 ) 2 Cl (aq) Complex Ions • The previously mentioned coordination complexes or metal complexes are referred to here as complex ions. • For the equilibrium Ag (aq) 2NH3 (aq) Ag(NH3 )2 (aq) • The formation constant, Kf, is 3 2 [Ag(NH ) ] Kf [Ag ][NH 3 ] Class Example • Calculate the concentration of Ag+ present in solution at equilibrium when concentrated ammonia is added to a 0.010 M solution of AgNO3 to give an equilibrium concentration of [NH3] = 0.20 M. Neglect the small volume change that occurs when NH3 is added Table Talk • Calculate [Cr3+] in equilibrium with Cr(OH)4when 0.010 mol of Cr(NO3)3 is dissolved in 1 L of solution buffered at pH = 10.0. The Kf for Cr(OH)4- is 8 x 1029 Class Example • The Ksp for AgI is 1 x 10-16 and Kf for Ag(CN)2- is 1 x 1021. Using these values, (a) calculate the molar solubility of AgI in pure water and (b) calculate the equilibrium constant for the reaction: 2 AgI(s) 2CN (aq) Ag(CN) (aq) I (aq) • and, (c) determine the molar solubility of AgI in a 0.100 M NaCN solution. Table Talk • The Kf of Ag(NH3)2 is 1.7 x 107 and the Ksp of AgCl (s) is 1.6 x 10-10. Calculate (a) the molar solubility of AgCl in pure water, (b) the equilibrium constant for the reaction: 3 3 2 AgCl(s) 2NH (aq) Ag(NH ) (aq) Cl (aq) • and, (c) the solubility of AgCl (s) in 0.50 M NH3 Collaborative Poster • Solve the two problems that are on the handout that you and your group received. • Explain all steps that you do so that someone who was not here could learn how to do these problems just from your poster. Free Energy • Recall that we calculated standard free energies (ΔGo), which were when all concentrations were 1 M and temperature was 298 K. • Most reactions occur at non-standard condition and we can calculate ΔG at this point with: G G RT lnQ o Free Energy and K • At equilibrium ΔG=0 and Q = K. Therefore: G G RTlnQ o 0 G RT lnK o G RTlnK o G RTlnK o Class Example • The equilibrium for the Haber process at 25 oC is N2 (g) 3H2 (g) 2NH3 (g) • What is the Kp for this reaction? N2 H2 ΔHfo (kJ/mol 0 0 NH3 -80.29 So (J/ (mol x K) 191.50 130.58 192.5 Table Talk • Calculate the value of the equilibrium constant for the following reaction: H2 (g) Br2 (l) 2HBr(g) H2 Br2 ΔHfo (kJ/mol 0 0 HBr -36.23 So (J/ (mol x K) 130.58 174.9 198.48 Relay Races 1.If K is calculated to be a value of 0.5 for a reaction at 25 oC, then is the reaction spontaneous? 2. If ΔG = 52 kJ/mol at 52 oC, then what is the value of K? 3. If K is determined to be 523 at 30 oC, then is the reaction spontaneous at these conditions? 4. Use the following standard free energy of formation for formic acid (HCO2). Calculate the Ka for the reaction: HCO2 H+ ΔGfo (kJ/mol -372.3 0 HCO2- -351.0 5. The Kf of Ag(NH3)2 is 1.7 x 107 and the Ksp of AgCl (s) is 1.6 x 10-10. Calculate the equilibrium constant and ΔG for the reaction: 3 3 2 AgCl(s) 2NH (aq) Ag(NH ) (aq) Cl (aq) Closing Time • Read 17.6, 19.7, and 20.2 • Homework: 17.60 17.61, 17.63, 17.64, 17.65, 19.79, 19.80, 19.81, 19.83