doc - Wits Structural Chemistry
advertisement

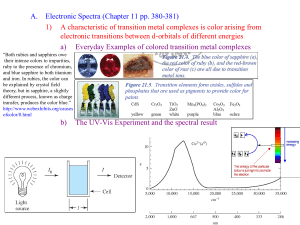
A/1 APPENDIX ELECTRONIC SPECTRA OF SOME TRANSITION METAL COMPLEXES One of the most important techniques for the study of compounds of the transition elements is electronic spectroscopy. A definition of a transition element is "an element which has partly filled d or f orbitals in some or all of its compounds" and it is these partially filed orbitals which lead to the electronic spectrum and, if the absorption is in the visible region of the spectrum, to coloured compounds In inorganic chemistry we can use electronic spectroscopy to: (i) determine the structure, geometry and even the absolute configuration of a compound. (ii) study equilibria in solution. (iii) study the kinetics of formation or decomposition of compounds, and (iv) determine the purity of our products. In this practical class you are required to run an electronic spectrum of your products to ensure that you have a pure sample, in the same way as any inorganic researcher would. In these practicals the spectra that are important are those of Co(III) (expts. I1 and I2), and Cr(III) (expt. I3), and inn these notes we will discuss the spectra of these ions. The electronic spectra of the first-row transition metal ions A full discussion of the spectra is outside the scope of the third year and, in fact, is only discussed at this University at the fourth year honours level. A/2 (1) Spectra of d3 complexes TABLE 1 Cr(H2O)63+ 406(15) 574(13) Cr(H2O)5Cl2+ 428(21) 607(16.4) cis- Cr(H2O)4Cl2+ 450(26) 635(18) trans- Cr(H2O)4Cl2 450(22) 635(24) Cr(NH3)63+ 350(32) 462(392) 350(52.5) 458(68.5) Cr(NH3)5H2O3+ 360(29) 479(34) Cr(NH3)5(OH)2+ 399(41) 507(43) 376(43.6) 515(38) 452(sh) cis-Cr(NH3)4(H2O)23+ 364(26) 493(36) trans- Cr(NH3)4(H2O)23+ 368(30) 476(20.6) 1,2,3 Cr(NH3)3(H3O)33+ 375(22) 518(34.5) 1,2,6 Cr(NH3)3(H2O)33+ 373(25.6) 503(25.6) trans Cr(NH3)2(H2O)43+ 382(18.5) 521(21) Ion Cren33+ Cr(NH3)5Cl2+ As can be seen from this table accurate measurement of band position and intensity can be a valuable method for the identification of your products. Note that the relative intensity of and can distinguish between the cis and trans isomers of Cr(H2O)4Cl2+ even though the band positions in the two ions are identical. (2) Spectra of d6 complexes In the spectra of low spin octahedral d6 ions up to five spin-allowed bands are theoretically possible, however, only two are normally observed, the rest occurring at too high energy. The two bands have been assigned as: Table 2 gives some of the spectra observed for Co(III) complexes. A/3 TABLE 2 Coen33+ 467(85) 340(72) Co(NH3)63+ 476 339 Co(NH3)5H2O3+ 485 340 Ion Co(NH3)5Cl2+ 535, 467(sh) Co(NH3)5Br2+ 550, 470(sh) - 529 390 617, 455 387 cis-Co(en)2Cl2+ trans-Co(en)2Cl2+ 363.5 You will notice that the band is fairly sensitive to the symmetry of the complex. In the purely octahedral Co(NH3)63+ one peak is observed but by lowering the symmetry to C4v in Co(NH3)52+, where X = Cl or Br, a shoulder appears. In trans Co(en)2Cl2+, two full bands appear instead of one. Spectra of octahedral and tetrahedral Co(II) One of the most interesting phenomena associated with Co(II), d7, is its ability to readily form either octahedral or tetrahedral complexes depending on the choice of ligands. A simple example is provided by CoCl2.6H2O, which gives the characteristic pink-red colour of octahedral Co(II) when dissolved in water but gives the deep blue colour that is often associated with tetrahedral Co(II) in acetone. The colour is, of course, not a good criterion of stoichiometry, although it can give an indication. The electronic spectrum can provide a definitive answer as to the stoichiometry. Both octahedral and tetrahedral Co(II) should theoretically give rise to three transitions. However, the band in octahedral complexes tends to be weak and is not often observed, whereas the band, if it does occur, would be in the infrared region due to the lower crystal field splitting in tetrahedral complexes (see Table 3 for spectra). Thus in the visible region the peaks observed are the bands in both geometries; the band in octahedral geometry occurring at somewhat higher frequency (lower wavelength) than those in tetrahedral geometry, hence the pink-red in the former and blue colours in the latter. However, there is another property of the spectra that provides an excellent method for distinguishing between the two geometries. The spectra of tetrahedral complexes are often of the order of 100 times more intense than those of octahedral. The reasons for this are too complex to go into here but it arises predominantly A/4 because of the lack of a centre of symmetry. The and of tetrahedral complexes is often structured and occurs as a multiple absorption due to vibrational structure among other reasons. Thus tetrahedral can be distinguished from octahedral both by the position and intensity of the visible band. TABLE 3 Co(II) d7 complexes Octahedral Co(H2O)62+ Co(NH3)62+ Co(pyO)62+ 1240(2) 1110(3) 1100(12) (625) 515(46) 474(8) 527(31) 501(31) 2092 699 1961 671 1539 721(639) 685(726) 636(504) 1199(172) 1075(155) 617(757) 607 Tetrahedral CoCO42 Co(etu)42+ Co(2Mepy)2NCS2 etu = ethylenethiourea REFERENCES A. Cotton and Wilkinson, 'Advanced Inorganic Chemistry', (3rd ed) 2. Lever, A.B,P., 'Inorganic Electronic Spectroscopy', Elsevier.