Lab 10
advertisement
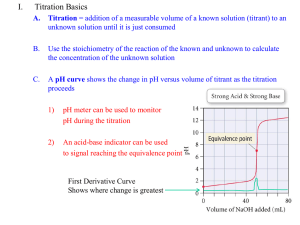
Analysis of Vinegar by Titration Lab 10 Outline • Purpose • Potentiometric Titrations • • • • • – Proticity – Equivalence – pKa Vinegar Titration Calculations Waste Following Lab Reminder pH Probe Calibration Purpose Students will use a pH probe to perform potentiometric titrations to determine the mass percent of acetic acid in vinegar and the pKa of acetic acid. Potentiometric Titrations Potentiometric titrations can be used to determine: • proticity (how many acidic hydrogens are donated to solution) • the amount or concentration of acid or base present (using MaVa= MbVb) • pKa (the “– log” of the acid dissociation constant) Proticity Acetic acid is a monoprotic weak acid that reacts with NaOH or KOH in a 1:1 ratio and produces a single sigmoidal curve. 12.00 11.00 10.00 9.00 8.00 pH 7.00 6.00 5.00 4.00 3.00 2.00 0.00 5.00 10.00 15.00 Volume, mL 20.00 25.00 Equivalence 20 18 16 14 12 10 8 6 4 2 0 0.00 12.00 11.00 10.00 9.00 8.00 7.00 6.00 5.00 4.00 3.00 5.00 10.00 15.00 Volume, mL 20.00 25.00 pH Derivative The equivalence point volume is determined by plotting a derivative curve of the titration curve. The steepest point on the derivative curve corresponds to the equivalence point volume. (Find the exact value on your spreadsheet!) Equivalence Point Volume • The concentration of acetic acid can be determined from: – the equivalence point volume of the base – the concentration of the base – the volume of the acid used in the titration • The equation to use is: – MaVa = MbVb pKa The pKa of acetic acid is the pH at the halfequivalence point volume of the titration, because: For a weak acid: HA H+ + A- and Ka = H A H A At the half-equivalence point, half the acid has been converted to its salt, so: [HA] = [A-] Ka = [H+] pKa = pH Vinegar Titration • Make the required dilution of vinegar. • Calibrate your pH probe. • Titrate the specified aliquots to obtain titration curves. • Determine the volume of base delivered at each time point, using the base delivery rate. • Graph the derivative of your titration curves following the instructions in the manual. • Make up a spreadsheet that will allow you to calculate the indicated values. Spreadsheet Time, s 0.01 35.95 60.24 79.47 103.54 142.46 151.31 187.66 222.13 268.71 pH Vol, mL 3.02 0.02 4.15 1.97 4.65 5.21 4.74 10.44 5.16 13.45 5.34 16.11 8.50 20.89 10.58 21.52 11.35 22.95 11.73 25.36 Deriv 0.001 0.000 0.003 0.000 0.002 0.010 18.750 1.960 0.001 0.000 pKa Half-equivalence Point Volume Highest derivative Equivalence Point Volume Calculations • At the equivalence point: moles of base = moles of acid (because they react in a 1:1 ratio) • Moles of base = (Eq Pt Vol base, L) x ([base], M) • Mass of acetic acid = (molar mass of acetic acid, g/mol) x (moles of acid, moles) • Mass of vinegar = (density, g/mL) x (Vol vinegar, mL) • Mass % = (mass of acetic acid / mass of vinegar) x 100% Safety Concerns • Reagents: Acetic Acid (1 N) Sodium Hydroxide (0.1 N) / Potassium Hydroxide (0.1 N) • Eye Contact: Irritation, tearing, redness, pain, impaired vision, severe burns and irreversible eye injury. • Skin Contact: Severe skin irritation, soreness, redness, destruction of skin (penetrating ulcers) . May cause sensitization and / or allergic reaction. • Inhalation: May cause coughing, serious burns, pneumonitis, pulmonary edema, and coma. • Ingestion: Toxic. Corrosive to mucous membranes. May cause perforation of the esophagus and stomach, abdominal pain, nausea, vomiting, diarrhea, general gastro-intestinal upset. Waste • Only solutions with a pH between 6 and 8 can go down the drain. • All other solutions need to go in the acid/waste container in the fume hood. Lab 11 Reminder • Lab 11 is next. pH Probe Calibration • Calibrate your pH probe according to the instructions in your lab manual. • Be careful not to break the bulb. • Be careful not to contaminate the buffer solutions. • Ask your instructor if you need help.





![CSUS - CH6A, [Mass percentage of acetic acid in vinegar] Instructor](http://s3.studylib.net/store/data/007937173_1-e0c351dc5daed812e8e6ea99de8c0e8a-300x300.png)



