Chapter 6 Slides - University of Iowa
advertisement

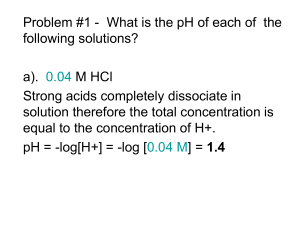
Chapter 6: Acid and Bases, Electrophiles and Nucleophiles I. Acid-Base Dissociation A. Water Acting as a Base H Ka' + O H A H Ka ' aA aH O 3 aHA aH 2O H O+ H H + - A [A ][H 3O ] yA yH3O [HA][H 2 O] yHA yH 2O Since for dilute solution the activity of water is constant: K a K a ' aH 2O [A ] y A a H 3O [HA] y HA [A ] pKa pH log pH log I [HA] 1 B. Water Acting as An Acid Kb' B + Kb' H OH aHO aHB aB:aH 2O + B H + - HO [HO ][HB ] yHO yHB [B :][H 2 O] yB: yH 2O Proceeding as before: K b K b ' aH 2O [HB ] y HB a HO [B :] y B: [HB ] pK b pOH log pOH log I [B :] 2 Since pKW = pH + pOH = 14 pKb = 14 - pKa Conclusion: stronger bases have lower pKb values, and their conjugate acids are weaker acids (higher pKa values). 3 II. Strengths of Oxygen and Nitrogen Acids Acid pKa % Dissociation in Water H2SO4 -4 99.999 CH3CO-OH 4.76 1.3 Ph-OH 10.0 3.2 x 10-4 H-OH 14 1.8 x 10-7 Me-OH 16 3.2 x 10-7 Electronwithdrawing groups have a large effect on the acidity of the OH function. 4 Acid +OH R C H +OH R C R +OH R C OR' +OH R C OH + R O R' pKa -10 -7 -6.5 -7 -3.5 Many important intermediates in organic reactions are strong acids. Reference: Advanced Organic Chemistry; 4th Ed.; March, J.; John Wiley & Sons: New York, 1992, pp. 250-252. H + R CH2OH2 -2 5 Acid pKa NH4+ 9.2 CH3NH3+ 10.6 (CH3)2NH2+ 10.8 (CH3)3NH+ 9.8 Pyridinium 5.2 4-Nitropyridinium 1.2 PhNH3+ 4.6 Ammonium ions are stronger acids, and therefore their conjugate bases weaker bases, than their oxygen analogs. 6 III. Leveling Effects of the Solvent The strongest acid that can exist in a solvent is the conjugate acid (lyonium species) of the solvent. H2SO4 + H2O pKa ~ -4 HCl + H2O H3O+ + HSO4- pKa = -1.7 H3O+ + Cl- pKa ~ -7 7 The strongest base that can exist in a solvent is the conjugate base (lyoxide species) of the solvent. NH2- + H2O (iPr)2N- + H2O NH3 + OH(iPr)2NH + OH- pKW = 14 pKa ~ 35-40 for neutral amines 8 IV. Rates of Proton Transfer A. Oxygen Acids kD A- + H3O+ HA + H2O kR Ka = kD/kR Rate constants for proton transfer from H3O+ to anionic bases are diffusion controlled. Values of kR are typically 1 to 5 x 1010 M-1 s-1. 9 pKa kD (s-1) kR (M-1 s-1) H2O 15.7 2.5 x 10-5 1.4 x 1011 D2O 16.5 2.5 x 10-6 8.4 x 1010 HF 3.2 7.0 x 107 1.0 x 1011 CH3COOH 4.8 7.8 x 105 4.5 x 1010 C6H5COOH 4.2 2.2 x 106 3.5 x 1010 p-NO2C6H4OH 7.1 2.6 x 103 3.6 x 1010 HA 10 Configuration diffusion of H3O+ and –OH: H H O H H +O H H H H H H H O O O H O O H H +O H H H H H H H O O H O H - H H H H O H O O O H H H O - H 11 B. Nitrogen Bases kR R3NH+ + HO- R3N + H2O kD Kb = kR/kD Ka = Kw/Kb pKa = 14 - pKb 12 R3N pKB pKa kR (s-1) kD (M-1 s-1) NH3 4.8 9.2 6 x 105 3.4 x 1010 MeNH2 3.4 10.6 1.6 x 107 3.7 x 1010 Me2NH 3.2 10.8 1.9 x 107 3.1 x 1010 Me3N 4.2 9.8 1.4 x 106 2.1 x 1010 Conclusion: Acid dissociation of ammonium ions is diffusion controlled. 13 V. Acidities of Carbon Acids Compound Class Example pKa Cyano Compounds CH3CN 25a Nitro Compounds CH3NO2 10.2a Sulphoxides CH3SOCH3 28a Ketones CH3COCH3 20a Esters CH3COOCH2CH3 26c Carboxylate Ions CH3COO- 33d Alkanes CH3CH3 CH3-CH=CH2 cyclopentadiene 50b 43b 15a Alkenes H2C=CH2 44b Alkynes H-C C-H 25b Aromatic Compounds CH3 43b, 40b a) Table 6.5, p 247 of book. b) Advanced Organic Chemistry; 4th Ed.; March, J.; John Wiley & Sons: New York, 1992, pp. 250-252. c) Amyes, T.L.; Richard, J.P. J. Am. Chem. Soc. 1996, 118, 31293141. d) Richard, J.P.; Williams, G.; O’Donoghue, A.C.; Amyes, T.L. J. Am. Chem. Soc. 2002, 124, 2957-2968. 14 A. Measurement of Weak Acidity Make a solution of two weak acids and add a substoichiometric amount of a strong base. Measure the equilibrium concentrations: Keq HA1 + A2- HA2 + A1- 15 Calculation of pKa values: [HA2 ][A1 ] yHA 2 yA1 I 2 K eq I1 [HA1 ][A 2 ] yHA1 yA 2 pKa (HA1 ) pH log pKa (HA2 ) pH log [A1 ] yA 1 [HA1 ] yHA 1 [A 2 ] yA 2 [HA2 ] yHA 2 pH log I1 pH log I 2 16 log K eq I2 pKa ( HA2 ) pKa ( HA1 ) log I1 pKa Keq 10 where pKa pKa ( HA2 ) pKa ( HA1 ) Medium pKa Range H2O/HO- 1 – 14 CH3OH/CH3O- 14 – 16 CH3SOCH3/ CH3SOCH2- 13 – 28 / NH2 - + NH Cs 18 – 32 17 B. Factors that Affect Carbon Acidity 1. Substituent Effects Carbon Acid pKa CH3CN 25 11 -5 CH2(CN)2 CH(CN)3 CH3NO2 CH2(NO2)2 CH(NO2)3 O CH3 CH3 C 10 3.6 0.1 20 Substituents stabilize conjugate base anion by resonance delocalization of negative charge. CH3 O O C C CH2 12.6 CH3 18 2. Aromaticity H H - + B: + BH + pKa = 15 CH3 CH CH2 + B: [CH2 CH CH2 ] - + BH+ pKa = 43 19 3. Stabilization by d-orbitals CHCl3 + B: Cl2C--Cl Cl2C=Cl- pKa = 25 R3P+-CH3 + B: R3P=CH2 R2S+-CH3 + B: R2S=CH2 R3P+-CH2R2S+-CH2- pKa ~ 30 R3N+-CH3 + B: R3N+-CH2- + BH pKa ~ 40 CH3-CH3 + B: CH3-CH2- + BH pKa ~ 50 20 4. s-Character of Carbon Hybrid Orbitals Carbon Acid % s-Character pKa H-CC-H 50 25 H2C=CH2 33 44 CH3CH3 25 50 21 C. Nitrogen Acids Acid NH3 pKa ~ 40 C6H5NH2 30 CH3CONH2 26 (H2N)2C=O 27 (H2N)2C=S 21 N-H bond tends to be more acidic than C-H bond due to higher electronegativity of N than C. 22 VI. Theories of Proton Transfer A. Eigen Model kd A-H + B k-d (A-HB) kp (A-H-B+) k-p k-d A- + H-B+ kd kd = 4N(rAH + rB)(DAH + DB)e 23 B. Marcus Theory GP Free Energy GR Gp‡ G‡ WP WR } G o p } G o Reaction Progress G‡ = Gp + WR ‡ 2 W G 1 R 4 ‡ G op 24 WR = work required to form encounter complex from reactants WP = work required to form the encounter complex in the reverse direction from products GR, GP = free energies of reactants and products, respectively, within the encounter complex G‡ = overall free energy of activation Gp‡ = free energy of activation for proton transfer within the encounter complex Go = overall equilibrium free energy of reaction Gpo = equilibrium free energy of reaction within the encounter complex 25 Derivation of the Marcus Theory Equation: Gp‡ = x2 = (x-1)2 + Gpo x2 = (x-1)2 + Gpo o 1 G p x 1 2 26 Since Gp‡ = x2: Gp‡ 1 4 G op 2 Therefore, when Gpo = 0: Gp‡ G‡int = /4 and = 4 G‡int Position of the transition state: x‡ = ½ + Gpo/8 G‡int 27 VII. Nucleophilicity and Electrophilicity A. BrØnsted Linear Free Energy Relationship A formal similarity is noted between proton transfer and nucleophilic displacement or nucleophilic addition: CH3CH2NH2 + H CH3CH2NH2 + CH3 + - CH3CH2N H3 + I I I + CH3CH2NH2CH3 + I 28 O CH3CH2NH2 + H O C + CH3CH2N H2 - C H BrØnsted equation for nucleophilic reactions: knuc = Gnuc Ka-βnuc Taking the log transform: log knuc = bnucpKa + log Gnuc = bnucpKa + C 29 B. Nucleophilic BrØnsted Plot for Stepwise Mechanism k obs k 1k 3 k 1k 3 / k 2 k 2 k3 1 k3 / k2 30 Since ki = Gi Ka-bi: k obs G123 K ba 2 b1 b3 1 G 23 K ba 2 b3 and log kobs = (b3+b1-b2) pKa + C123 – log(1 + G23Kab2-b3) or log kobs = (b3+b1-b2) pKa + C123 – log(1 + G2310(b3-b2)pKa) The equation is nonlinear because of the last term. 31 Special Cases: 1. k1 is rate-determining: k3 >> k2 k3/k2 >> 1 T- kobs = k1 G log kobs = β1pKa + C1 A + Nu P Reaction Progress 32 2. k3 is rate-determining: k3 << k2 k3/k2 ~ 0 - T G kobs = k1k3/k2 log kobs = (β3+β1-β2)pKa + C123 A + Nu P Reaction Progress 33 Example 1: reactivity of various imidazoles toward p-nitrophenyl acetate 2 Slope = b = 0.8 Reference: Bruce and Lipinski, J. Am. Chem. Soc. 1958, 80, 2265. 1 log k1 0 -1 -2 3 4 5 6 7 8 9 pKa 34 High sensitivity of rate constant to basicity of nucleophile is consistent with a late transition state with appreciable +-charge on bonding atom of nucleophile: ‡ Y H CH3 +N d N C O NO2 Od Appreciable bond making 35 Example 2: Acetylation of Substituted Pyridines Y + N CH3 O O C C O k1 CH3 k2 + N log kN (M-1 s-1) - C OAc Y CH3 T 4 O + O + N CCH3 k3 Y - + CH 3CO2 b = 0.2 Castro and Castro, J. Org. Chem. 1981, 46, 2939-2943. 2 b = 1.0 0 -2 2 4 6 pKa 8 10 36 The nonlinear BrØnsted plot is proof of an intermediate: pKa < 6 Breakdown of T± is rate-determining. kobs = k1k3/k2 βobs = β3 + β1 - β2 pKa > 6 Formation of T± is rate-determining. kobs = k1 βobs = β1 pKa ~ 6 Both k1 and k3 are rate-determining. k obs k1k 3 k 2 k3 37 O + N - C O CCH3 Y CH3 T O Leaving group abilities match for CH3CO2- (pKa = 4.8) and YPyr when pKa of YPyrH+ is 6.1. + Conclusion: A nonlinear BrØnsted plot requires a mechanism with at least one intermediate. Caveat: The converse, that a linear BrØnsted plot requires a concerted mechanism, is not true. 38 Example 3: An unambiguous test for concertedness. Use nucleophiles of the same structural class as the leaving group. O O - O OCCH3 OCCH3 + Y O + O2N Y O2N 39 - Prediction for a stepwise mechanism: O O O - OCCH3 + Y O2N O k1 Y - C CH3 O k2 TNO2 k3 k3 = k2 when pKa = 0 O O - OCCH3 + O2N Y 40 O O2N O - C CH3 O OH pKa of is 6.98 O2N , if reaction is stepwise, BrØnsted plot must have a break at pKanuc = 7. TNO2 41 pKa = 0 - log knuc Od CH3 C OC 6H4NO2 - OC 6H4Y d - d O CH3 d- C OC 6 H4 NO 2 OC 6 H4 Y 5 6 7 8 9 10 pKa 42 What is observed? log kArO- (M-1 s-1) 1 0 Ba-Saif, Luthra & Williams J. Am. Chem. Soc. 1987, 109, 6362-6368 pKa = 0 -1 -2 b = 0.75 -3 -4 5 6 7 8 9 10 11 pKa(ArOH) 43 For the equilibrium: O O - OCCH3 O OCCH3 Keq + Y O + O2N O2N Y log Keq = C + βeq pKanuc β = 1.7 α = βnuc/βeq = 0.44 α is a measure of the position of the transition state on a More O’Ferrall-Jencks diagram: 44 - O - YArO O OAr MeCOArY + ArO Me ‡ O O - + YArO + ArO C MeCOAr + YArO O2N + Me ‡ d- CH3 O C O 0.44 O 0.44 d- NO2 45 C. Hard and Soft Acids and Bases Various observations indicate that the correlation of nucleophilicity with basicity, as measured by conjugate acid pKa values, is not universal: 1. 1. BrØnsted analysis degrades when nucleophiles of different structural classes 2. are used. 2. HI is a very strong acid (pKa = -9) whose conjugate base is nonetheless a strong nucleophile: O CH3 + H I + O CH3 + I - H OH + CH 3I 3. I- is an example of a soft Lewis base, and methyl is an example of a soft Lewis acid. 4. Hard-hard interactions and soft-soft interactions are stronger than hard-soft interactions. 46 D. Energetics of Nucleophile-Electrophile Interactions qn qe 2(c n ceb)2 E er E HOMO E LUMO qn and qe are charges on nucleophile and electrophile, respectively. cn and ce are orbital coefficients of nucleophile HOMO and electrophile LUMO, respectively. β is the resonance integral. EHOMO = energy of nucleophile HOMO ELUMO = energy of electrophile LUMO Electrostatic term: important for interactions of hard acids with hard bases Orbital interaction term: important for interactions of soft acids with soft bases 47 Bases (nucleophiles) Type Hard Intermediate Soft Species Examples small halide anions F-, Cl- oxygen nucleophiles H2O, ROH, HO-, RO-, ROR, MeCO2-, SO42-, PO43- amine nucleophiles RNH2, H2NNH2 larger halide anions Br- nitrogen nucleophiles C6H5NH2, C5H5N, N3- oxygen nucleophiles NO2-, SO32-- sulfur nucleophiles RSR, RSH, RS- phosphorus nucleophiles R3P, (RO)3P, R3As carbon nucleophiles CN-, CO, R-, C2H4, Ar others H -, I 48 Acids (electrophiles) Type Hard Intermediate Soft Species Examples high charge/radius cations H+, Li+, Mg2+, Ca2+, Al3+, Cr3+, Ti4+, I5+ group II species Be(CH3)2 group III species Al(CH3)3, BF3, B(OR)3 H-bond donors ROH, HOH, RNH2, RNH3+ moderate charge/radius cations Fe2+, Cu2+, Zn2+, Pb2+, Sn2+, Co2+, Ni2+ carbocations (CH3)3C+, ArH+ low charge/radius cations Cu+, Ag+, Au+, Hg+, Hg2+, I+, Br+, RO+ carbon electrophiles RL, ArL (L = nucleofuge) diatomic halogens I2, Br2, ICN radicals O, Cl, Br, I, RO 49 E. Quantitative Measures of Hardness and Softness Ionization potential measures EHOMO. Electron affinity measures ELUMO. Frontier Orbital Energies for Lewis Acids and Bases Base EHOMO (kJ) Acid ELUMO (kJ) H- -711 Hg2+ -448 I- -801 Ag+ -272 SH- -829 Na+ 0 CN- -847 H+ 40.5 Br- -889 Li+ 47.3 Cl- -959 Fe3+ 66.5 HO- -1008 Ca2+ 225 H2O -1032 F- -1175 soft hard 50 Reactivity Trends: 1. Hg2+ (ELUMO = -448 kJ mol-1, soft electrophile) HS- > CN- > Br- > Cl- > HO- > FReactivity parallels EHOMO of nucleophile. 2. Ca2+ (ELUMO = 225 kJ mol-1, hard electrophile) HO- > CN- > HS- > F- > Cl- > Br- > IReactivity parallels pKa of conjugate acid of nucleophile. 51 F. Structure-Nucleophilicity Correlations 1. Swain-Scott LFER log kNu/k0 = sn n nucleophilicity parameter s = sensitivity of studied reaction (s = 1 for reaction in H2O) Reference reaction: Nu:- + CH3Br NuCH3 + Br52 Nucleophile n MeOH n0 0 H2 O 0 AcO- 2.72 F- n values are for reactions in H2O. 4.3 2.7 n0 values are for reactions in MeOH. More values in Table 6.10 Cl- 3.04 4.33 Br- 3.89 5.79 I- 5.04 7.42 SCN- 4.77 6.7 C6H5NH2 4.49 5.7 Correlations best for nucleophilic displacements at saturated carbon. 53 2. Edwards “Oxybase” Equation log kNu/k0 = aEN + bHN EN soft nucleophilicity (based on oxidation potentials) HN hard nucleophilicity (based on pKa values) k0 = rate constant for reaction with water EN and HN parameters are tabulated in Table 6.10. Examples: Nu:- + CH3Br Nu:- + HO-OH NuCH3 + BrNuOH + HO- a = 2.50 b = 0.006 a = 6.22 b = -0.43 54 3. Ritchie Nucleophilicity Parameters log kNu/k0 = N+ Features: ● Not a LFER. ● Provides a scale of nucleophilicities for anion/solvent systems. ● Reference system is H2O. ● Works for reactions with carbocation and carbonyl carbons. 55 Ritchie Nucleophilicity Parameters System N+ H2O CH3OH 0.0 0.5 Conclusions: CN-/H2O CN-/CH3OH CN-/DMSO CN-/DMF 3.8 5.9 8.6 9.4 Softer Lewis bases (nucleophiles) are more reactive. N3-/H2O N3-/CH3OH 5.4 8.5 PhS-/CH3OH PhS-/DMSO 10.7 13.1 HO-/H2O CH3O-/CH3OH 4.5 7.5 Hydroxylic solvents impede nucleophilic reactivity. 56 G. Relationship Between Nucleophilicity and Nucleofugacity 105 k2 (M-1 s-1) I- + EtI* EtI + I*- Ratio 6000 1440 Pyr + EtI EtPyr+ + I- 4.17 I- + EtBr EtI + Br- 195 269 Pyr + EtBr EtPyr+ + Br- 0.725 57 CH3 Nu C H - X H 1 1 BCX CH3CH2Nu 0 1 BCNu + X - BCNu 0 1 Nu: + CH3CH2X 0 0 BCX - Nu: + X + CH 3CH2 58 H. α-Effect Nucleophiles ● Hydrazines, hydroxylamines, peroxide anions ● Unusually strong nucleophiles in relation to their weak basicity ● Lone-pair repulsions raise EHOMO. Example: HOO - + -O2CCH2 Br - O2CCH2 OOH + Br - + -O2CCH2 Br - O2CCH2 OH + Br HO - - kHOO-/kHO- = 20 pKa of H2O = 15.7 pKa of H2O2 = 11.6 59