lecture notes-growth kinetics-web
advertisement
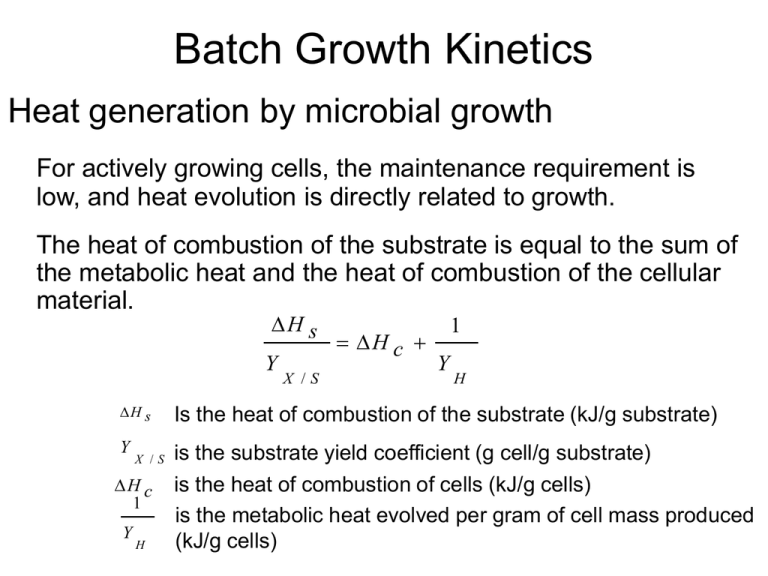
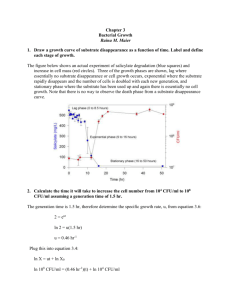
Batch Growth Kinetics Heat generation by microbial growth For actively growing cells, the maintenance requirement is low, and heat evolution is directly related to growth. The heat of combustion of the substrate is equal to the sum of the metabolic heat and the heat of combustion of the cellular material. H s Y X /S H c 1 Y H H s Is the heat of combustion of the substrate (kJ/g substrate) Y is the substrate yield coefficient (g cell/g substrate) is the heat of combustion of cells (kJ/g cells) is the metabolic heat evolved per gram of cell mass produced (kJ/g cells) X /S H c 1 Y H Batch Growth Kinetics Heat generation by microbial growth Re-arrange the above equation: Y H Y X /S H s Y X /S H c The higher degree of oxidation of the substrate has lower amounts of heat released: YH Glucose: 0.42 g/kcal ethanol: 0.18 g/kcal Methanol: 0.12 g/kcal Methane: 0.061g/kcal Batch Growth Kinetics Heat generation by microbial growth Re-arrange the above equation: 1 Y H s Y H H c X /S The total rate of heat evolution in a batch fermentation QGR is Q X GR V L net X 1 Y (kJ/h) H is the cell mass concentration (g/l); V L is the liquid volume (l) Heat removal: by circulating cooling water through a cooling coil or cooling jacket in the fermentation. Quantifying Growth Kinetics • Structured model: recognizing the components in cells in response to the environment. • Unstructured model: assuming fixed cell composition (balanced-growth). • Segregation model: segregating the culture into individual units (cells) that may differ from each other. • Nonsegregation model: cells are the same in the culture. Quantifying Growth Kinetics • Unstructured model: assuming fixed cell composition. Applicable to balanced-growth condition: - exponential growth phase in batch culture - single-stage, steady state continuous culture - cell response is fast compared to external changes - the magnitude of the external changes is not too large (e.g. 10%-20% variation from initial conditions). • Nonsegregation model: assuming all cells are the same in the culture. Satisfactory under most circumstances. Quantifying Growth Kinetics • Monod equation: unstructured and nonsegregation model Assumption: - a single enzyme system with Michaelis-Menten kinetics is responsible for uptake of substrate S, and the amount of that enzyme or its activity is sufficient low to be growth-rate limiting. - the relationship of specific growth rate to substrate concentration assumes the form of saturation kinetics. - a single chemical species is growth-rate limiting while changes in other nutrient concentrations have no effect. Applicable when growth is slow and population density is low. Monod type saturation growth kinetics Quantifying Growth Kinetics • Monod equation: when applied to cellular systems, the gross specific growth rate g (1/time) g m S KS S m is the maximum specific growth rate (1/time). Ks is the saturation constant or half-velocity constant (g/l) when the substrate concentration S>> Ks g m (exponential growth phase), When endogenous metabolism is unimportant, g net Cells Growth in Continuous Culture Continuous culture: fresh nutrient medium is continually supplied to a well-stirred culture and products and cells are simultaneously withdrawn. At steady state, concentrations of cells, products and substrates are constant. In batch culture: the culture environment changes continually. growth, product formation and substrate utilization terminate after a certain time interval. A continuous-culture laboratory setup medium Products, cells Cells Growth in Continuous Culture Ideal Chemostat: same as perfectly mixed continuous-flow, stirred-tank reactor (CFSTR). - Control elements: pH, dissolved oxygen, temperature - Fresh sterile medium is fed to the completely mixed and aerated (if required) reactor. - Suspension is removed at the same rate. - Liquid volume in the reactor is kept constant. Ideal Chemostat Subscript 0 denotes the parameters at the feed medium A material balance on the cell concentration around the chemostat yields, FX 0 FX V R g X V R k d X V R dX dt F is the volumetric flowrate of nutrient solution (l/h); VR is the volume (l) (constant); X is the cell concentration (g/l); P is the extracellular product (g/l); µg and kd are growth and endogenous rates, respectively (h-1). Cells Growth in Continuous Culture Ideal Chemostat Re-arranging the previous equation yields: DX 0 ( g k d D ) X dX dt D=F/VR, is dilution rate (1/time), the reciprocal of residence time. Usually, the feed media are sterile, X0=0, if g k d At steady state, dX/dt=0, then µg = D (kd ≈0) (manipulating the growth rate) g D m S KS S (using Monod equation) Where S is the steady state limiting substrate concentration (g/l) Cells Growth in Continuous Culture Ideal Chemostat Washed out: If D is set at a value greater than µm , the culture cannot reproduce quickly enough to maintain itself. When D ≤ µm , S K S D m D A mass balance on limiting substrate in the absence of endogenous metabolism yields 1 FS 0 FS V R g X M Y X / S VR q p X 1 Yp/s VR dS dt qp is the specific rate of extracellular product formation (g P/g cells-h) Yp/s is the product yield coefficient (g P/g S). Cells Growth in Continuous Culture Ideal Chemostat When qp=0, at steady state, dS/dt=0, then D (S0 S ) g X M Y X /S Since µg = D (kd ≈0), the above equation becomes X Y M X /S (S0 S ) Then X can be expressed by (qp=0 ,kd ≈0, Monod equation), X Y M X /S (S0 S ) Y M X /S (S0 KsD m D ) Cells Growth in Continuous Culture Ideal Chemostat When qp=0, kd ≈0, X0=0, Monod equation is applied, at steady state, m S g D KS S X Y M X /S X Y S M X /S K S D m D (S0 S ) (S0 S ) Y M X /S (S0 KsD m D ) Determination of Monod Parameters g 1 g S g m S KS S KS 1 m S KS m 1 m S (Hanes m g m K S ( Lineweaver g - Burk) - Woolf) (Eadie - Hofstee) s Chemostat: µg=D, D~S, reliable, constant environment, operation may be difficult. Batch: X, S, t → lnX ~ t , get µm (slope) from data in exponential phase X ln X0 μ net t μ m t Slope of cell cnc. verse time → µg ~ S, use the above plot to get Ks. Parameters need to be checked carefully.