Kinetics o f B i o l o g i c a l
Processes
Assoc. Prof. Dr. Mohd Razif Harun
Learning Outcomes
v
v
v
Kinetics of cell/biomass growth
Kinetics of product formation
Kinetics of substrate utilization
v
To propose a bioreactor system for plant,
animal cell cultures
To select the bioreactor operation modes for
fermentation and cell growth
v
CULTURE DENSITY INCREASE
CELL DIVISION
What do you
understand about
growth of cells?
3
Photosynthesis -need co2 n water to form sugar (c6h12o6)
Growth: basic concepts
Precursors
breaking down
Anabolism = biosynthesis
Catabolism = reactions to
recover energy (often ATP)
Growth of Cells
1. Replication and change in cell size
2. Cells grow under physical, chemical and
nutrional conditions
carbon
hydrogen
nitrogen
oxygen
phosphorus
sulfur
3. Cells extract nutrients from the medium and
convert them into biological compounds
4. Nutrients used for - energy, biosynthesis, product
formation
5. Result of nutrient utilization, microbial mass
increases with time:
5
5
a)
(w1-w2)/v
w1=before dry
w2=after dry
1. Dry weight (filtration or centrifugation)
2. Optical density (OD) (light scattering, nm)
calibration curve, generate using UV-Vis, conc vs absorbance, estimate unknown
C"# F$
/001)2/31(# 4.%# 5)6(7 281,8(%
/001)2/,).9
•
5(,+.7#
.4# .< 1/,)1(##
0
;8= (97(7#
.= 1)7# # " ? = < >
)% $ (7#/,##
. # B 4.%# CD#.+# E ; % 8
A
@
7%&# (' )*,+ #
5(/;(%8 7
•
•
/001)2/31(# 4.%# 08(%
•
/ 0
; (2,%.5(,(%
•
);#
,> )3 % 8 7),&?
)9(60(9;)I(H#
2/1)3/% ,).9#
,. 7%& J (' )*,+
•
281,8(%
); 501( 5(,+.7
28I% (#
,.# (% 1/,(;#
F$#
6
6
non-chemistry ppl use
(b) Indirect method
Measure biomolecule concentration and correlate to dry
cell mass concentration.
• Intracellular components of cells – RNA, DNA, protein, ATP
• Measurement of substrate consumption and/or product
formation during the course of growth
7
7
8
9
9
• Adaptation of cells to a new environment
10
10
11
11
miu, specific growth
rate
x=2xo
•
•
Known as late log phase
Slowed growth due to
1. Nutrient depletion – 1 or more
2. Waste accumulation – toxic byproducts
3. Unbalanced growth and metabolism
shifts for survival
14
14
secondary metabolites
endogenous metabolism
lag phase, miu=0
log phase,refer eqn
Known as decline phase
Cell lysis (spillage) may occur – intracellular nutrients released into
the medium – used by living organisms during stationary phase
Growth can be re-established by transferring to fresh media
At the end of the stationary phase:
•
•
Total nutrient depletion and toxic product
accumulation
Death phase begin
16
16
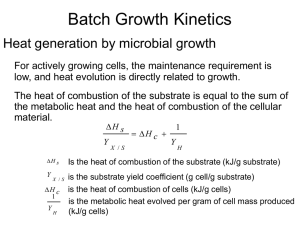
Stoichiometric Coefficients for growth
Yield coefficients
-ve because substrate decrease with time
Unit = g dry cells/g substrate consumed
Problem Example
x/xo in(x/xo)
Kinetics of product formation
Growth
Associated
Products
Non-growth
Associated
Product
Mixed Growth
Associated
Product
18
Kinetics of product formation
1. Growth associated products: products appear
simultaneously with cells in culture
e.g., production of a enzyme kinetics
19
Kinetics of product formation
2. Non growth-associated: product formation occurs during
Stationary Phase when the growth rate is zero
• Specific rate of product formation is constant
Secondary metabolites –antibiotics (e.g., penicillin)
20
Kinetics of product formation
3. Mixed-growth associated products: products
appear during slow growth and stationary phase
Specific rate of product formation:
e.g., lactic acid fermentation, xanthan
gum, & some secondary metabolites.
21
Growth Kinetics MODEL : Monod Equation
Monod equation is a kinetic model which
describes microbial growth as a functional
relationship between the specific growth
rate and an essential substrate
concentration
Similar to Michaelis-Menten, Langmuir-Hinshelwood (HougenWatson)
•
This is the most common kinetic model for cell growth.
Growth Kinetics MODEL : Monod Equation
Similar to Michaelis-Menten, Langmuir-Hinshelwood
(Hougen-Watson)
• Assumes that a single enzyme system is responsible for the uptake of
substrate (S), and that S is limited (growth-dependent variable).
• This is the most common kinetic model for cell growth.
22
Monod equation
1
𝑑𝑋
𝜇 𝑚𝑎𝑥 𝑆
×
=𝜇 =
𝑋 𝑑𝑡
𝐾𝑠 + 𝑆
Where:
X = concentration of bacteria degrading the substrate.
µ = Specific growth rate of bacteria
µmax = Maximum value of specific growth rate
S = Concentration of limiting substrate or nutrients
Ks = Saturation constant, equal to the concentration of substrate giving
growth rate of µmax
Application and importance
If the concentration of S is
reduced, the population
growth rate will decrease. If
concentration of S increases
to a specific limit where
growth rate is maximum, then
S is no longer regarded as a
limiting factor.
Figure 1 is a graphic representation of Monod’s equation
(Von Sperling and De Lemos Chernicharo 2005).
When Ks = S the term S/(Ks +
S) becomes half (1/2) and the
growth rate becomes equal to
½ maximum rate.
(Von Sperling and De Lemos Chernicharo 2005).
27
The aim of Monod was to establish that bacterial growth
rate was a function of the substrate concentration increase
to a certain level where the rate of growth becomes
constant with increased substrate concentrations.
Problem Example
Time (d)
Biomass Concentration
(mg/l)
0
50
1
68
2
91
3
123
4
166
5
224
Take the population vs. time data in table above
a) calculate the maximum specific growth rate
b) calculate the maximum specific growth rate that would be required
using the Monod model with a substrate concentration of
25 mg/L and a Ks of 5 mg/L.
29
quiz 1
15 question (MCQ) - 30 min
ch1 n ch2
test 1
3 questions (essay type) -1.5 hrs
with calculation
ch3 n ch4
can use Excel to plot
graph