Deactivation kinetics……Half life….
advertisement

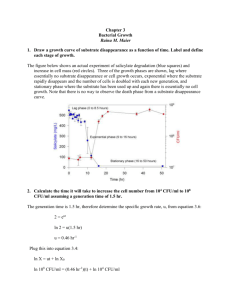
v k e 3 v k e 3 k Ae 3 Ea / RT de a dt k d ea e a e 0 exp( k d t ) k d A exp( E / RT ) d d Deactivation kinetics………… • The value of vmax for enzymatic reaction depends on the amount of active enzyme present….. v k e m 3 a k e exp( k t ) 3 0 d v m0 v v m m0 exp( k t ) k e initial value of v 3 0 before d m deactivati on occurs Deactivation kinetics……Half life…. • Half life is the time required for half the enzyme activity to be lost as a result of deactivation i.e. if ea = e0/2 ==> t=t1/2 t 1/ 2 ln 2 k d • For a batch reactor, v s v m K M ds s dt s s K K ln M exp( k t ) d M s s 0 s s t ds v exp( k t ) dt m0 s 0 m0 dt K s M v ds (s s) 0 d 0 v m0 k d exp( k d t ) 1 k s exp( k t ) 1 (s s) K ln v s d 0 d M 0 m0 k s k t ln 1 (s s) K ln v s d 0 d M 0 m0 k s t ln 1 (s s) K ln k v s 1 d 0 M d m0 0 Without deactivation……………… v s v max K M s ds dt tb K K M ln s M s so s ds v max so (s0 s) K M ln s 1 v max dt ( s 0 s ) v max t b tb 1 v max 1 K M ln 1 X A s0 X A • 13.1 Economics of batch enzyme conversion An enzyme is used to convert substrate to a commercial product in a 1600L batch reactor. Vmax for the enzyme is 0.9g/L.h; Km is 1.5g/L. Substrate concentration at the start of the reaction is 3g/L; according to the stoichiometry of the reaction, conversion of 1g substrate produces 1.2g product. The cost of operating the reactor including labor, maintenance, energy and other utilities is estimated as $4800/day. The cost of recovering the product depends on the extent of substrate conversion and the resulting concentration of product in the final reaction mixture. For conversions between 70% and 100%, the cost of Down Stream Processing can be approximated as C=155-(0.33X) where C is the cost in $ per kg of pdt treated and X is the % substrate conversion. The market price for the product is $750/kg. Currently the enzyme reactor is operated with 75% substrate conversion; however it is proposed to increase this to 90%. Estimate the effect this will have ion the economics of the process: • Total Expenditure = Upstream cost + Downstream cost • Profit = Price of product - Expenditure • Given S0 = 3 g/L For 75% conversion…. • Since 75% is converted…….Sf = (10.75)3=0.75 g/L • Amount of substrate consumed per batch = (S0-Sf)x(volume of rector) • = (2.25 g/L) 1600L = 3600 g • Therefore, tb = 4.81hrs • Given that 1 g substrate produces 1.2g of product • Therefore, 3600g of substrate produces…..4320 g product • 4320 g pdt is produced in 4.81 hrs……..therefore for ONE day i.e 24hrs….. 21555 g of product is produced • Upstream cost = 4800 $ /day • Downstream cost, C=155-(0.33X) =C= 130.25 $ /kg pdt • Downstream cost = (130.25 $ /kg pdt)(21.55kg pdt day) = 2806.88 $ /day • Total expenditure = 4800+2806.88= 7606.88 $ /day • Price of product = 750 $ / kg • = (750 $ / kg)(21.55 kg/day) = 16,163 $ /day • Therefore, Profit = Price of pdt Expenditure Profit = 8556 $ • For 90% conversion…. • Since 90% is converted…….Sf = (1-0.9)3=0.3 g/L • Amount of substrate consumed per batch = (S0Sf)x(volume of rector) • = (2.7 g/L) 1600L = 4320 g • Therefore, tb = 6.837 hrs • Given that 1 g substrate produces 1.2g of product • Therefore, 4320 g of substrate produces…..5184 g product • 5184 g pdt is produced in 6.837 hrs……..therefore for ONE day i.e 24hrs….. 18197.455 g of product is produced • For 90% conversion……the profit is only 6556 $/day • Therefore…..first method is more economical.