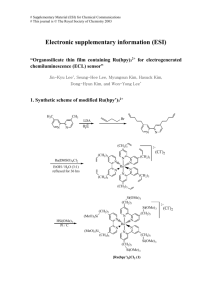
Synthesis of Modified Heme Protein Cofactors
advertisement
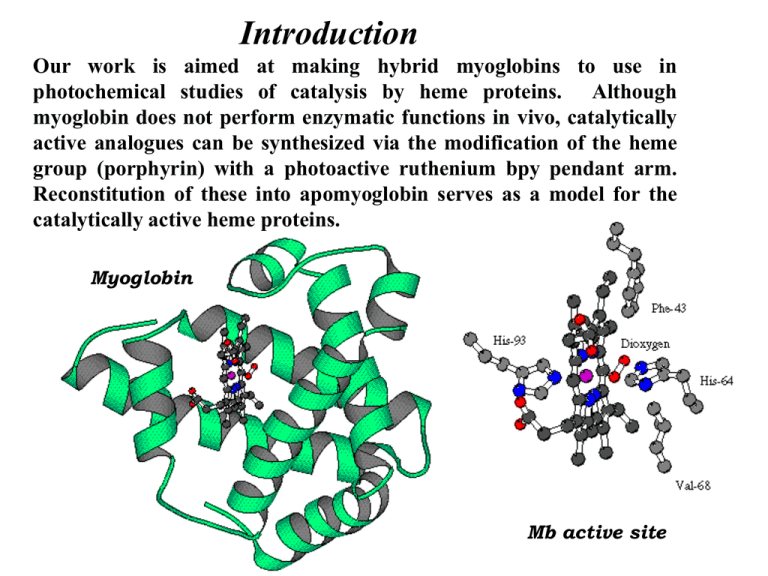
Introduction Our work is aimed at making hybrid myoglobins to use in photochemical studies of catalysis by heme proteins. Although myoglobin does not perform enzymatic functions in vivo, catalytically active analogues can be synthesized via the modification of the heme group (porphyrin) with a photoactive ruthenium bpy pendant arm. Reconstitution of these into apomyoglobin serves as a model for the catalytically active heme proteins. Myoglobin Mb active site Synthesis of Bpy Pendant Arm O (CH2)7Br LDA, Br(CH)6Br •Using this method, we can systematically vary the length and composition of the pendant arm. N (CH2)7 N O THF O DMF 85% N O KN 87% N N N N Conc. HCl Reflux, 10 hours (CH2)7NH2 (CH2)NH2 50% NaOH/sec-BuOH Free base 84% N N N N Synthesis of Protoporphyrin IX Derivative NH N CO 2Na PCl5, EtOH THF N HN NH sep. on SiO 2 35% CO2Na N CO2Et •This protects one proprionate which will be nessasary to help stabilize our reconstituted protein. N HN CO2H The Amide Coupling Reaction NH N N (CH2)NH2 DECP, (Et)3N THF Reflux 48 hours + HN NH 60% N N N HN N N CO2 Et CO2H CO2 Et Using UV-visible spectroscopy, we can determine when the C7PP or RuC7PP has been synthesized. CO NH(C H2)7 C7PP Base Hydrolysis NH N N HN N sep. on Al 2O3 Ru (bpy )2Cl2 CO2 H 1N NaOH THF CONH(C H2)7 Ru(bpy) 2CO 3 MeOH, H2O 75% N 1.8 400 nm 1.6 RuC7PP 1.4 1 288 nm RuC7PP C7PP 0.8 0.6 0.4 0.2 Wavelength (nm) 600 550 500 450 400 350 300 0 250 Absorbance 1.2 For the C7PP, there should be a 1 to 5 ratio between the peaks at 288 and 400 nm. The peak at 288 nm corresponds to bipyridine, and the one at 400 nm represents porphyrin. The spectrum of RuC7PP will have an increase for the 288 nm peak, increasing the aforementioned ratio to 3 to 5, as seen. N Metallation of Heme Cofactor NH FeCl 2 DMF Reflux, 4 hours N sep. on LH20 N 85% N N Fe HN N Cl N N N Ru(bpy)2Cl2 N CONH(CH2)7 CO2H Ru(bpy) 2Cl2 CO2H CONH(CH2)7 N RuC7FePP 1.2 400 nm RuC7FePP 1 288 nm 0.6 550 nm 0.4 650 nm 0.2 Wavelength (nm) 75 0 70 0 65 0 60 0 55 0 50 0 45 0 40 0 35 0 30 0 0 25 0 Absorbanc e 0.8 •A UV-visible spectrum was taken to verify the incorporation of iron into the porphyrin of RuC7PP. On the spectrum to the left, it can be seen that there are only two Q bands. This is diagnostic of the metallated porphyrin. The two Q bands are peaks at 550 and 650 nm. Unmetallated porphyrins, eg. RuC7PP and C7PP, have four Q bands. Preparation of Apomyoglobin • The synthesis of apomyoglobin occurs in a three step process: 1. Extraction of Native Heme 2. Dialysis 3. Lyophilization 4oC Myoglobin Apomyoglobin The reconstitution of the altered heme is done by mixing it with the apoprotein, and purifying the reformed holoenzyme by chromatography Molecular Model of RuC7FePP myoglobin Molecular Model of Hybrid Mb Characterization of the hybrid by UV Spectroscopy •When reconstituted into apomyoglobin, the UV-visible spectrum resembles what one would expect from a coupling of Ru(bpy)32+ and FeIII myoglobin. The soret shifts from 400 nm to 409 nm when reconstituted into protein. 0.8 Visible spectra of the hybrid Myoglobin 0.6 RuC7Mb Mb Ru(bpy)32+ 0.4 ca. 4 uM 0.2 0 300 400 500 600 700 Wavelength (nm) OD Nanosecond Transient Absorption Spectroscopy Time sample Monochromator Beamsplitter Termination Probe Light LASER Photoinduced nanosecond transient absorption FeIII Soret @ 410 nm FeII Soret @ 430 nm 0 1 2 3 Time (us) 4 0 1 2 3 Time (us) 4 •Using nanosecond transient absorption measurements of the heme soret bands in the hybrid Mb we can follow the fast electron transfer between Rubpy and the FeMb. •The transient traces illustrate the back electron transfer, kbet, regenerating the FeIII/Ru2+ state is 2 x 107 sec-1 Fe III *Ru 2+ h Fe III Ru 2+ kfet k fet Fe II Ru 3+ kbet hv Fe III Ru 2+ (byp) 3 Myoglobin k - b et Future Work his 52 During the next year we will attempt to reconstitute the RuC7FePP heme cofactor into CcP. In myoglobin the active site is located on the edge of the protein whereas in CcP one of the propionate groups is blocked and the other is recessed from the periphery of the protein. Due to the topographic differences in the active sites, it is probable that we will need to alter the length and characteristics of the pendant arm in order for successful reconstitution. trp 51 his 175 CcP Active Site Other Possible Pendant Groups: -Re(bpy)(CO)3L (L= Cl-, Br-, pyridine) -Methyl viologen N NR' trp 191 Acknowledgments • Farmer Group • Greg Qushair • David Khandabi • Phuong Do