Genetics Protein Project

Myoglobin
By Emily Espinosa
Amino Acid Sequence
Structure
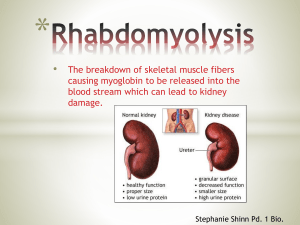
Myoglobin is a single-chain, iron-containing protein found in muscle fibers, structurally similar to a single subunit of hemoglobin.
Human myoglobin has 153 amino acid residues in a highly folded and compact structure with eight separate and distinct alpha helical secondary structures.
Function
Its main function is to carry oxygen molecules to muscle tissues.
It comprises of a single polypeptide chain and a heme
(an iron containing molecule that binds with protein) group, which reversibly binds a molecule of oxygen.
This is only relinquished at relatively low external oxygen concentrations, e.x. during strenuous exercise when muscle oxygen demand outpaces supply from the blood. Therefore, myoglobin acts as an emergency oxygen store.
Uses
It is used by humans and any other animal with muscles.
Pics
Why I chose myoglobin
It sounded interesting and its important for our muscles.
Other facts
It is found abundantly in the tissues of diving mammals, e.x., the whale, the seal, and the dolphin. High concentrations of myoglobin in these animals presumably allows them to store sufficient oxygen to remain underwater for long periods.
Work Cited
http://www3.interscience.wiley.com:8100/legac y/college/boyer/0471661791/structure/HbMb
/hbmb.htm
http://www.protasis.com/images/MbRib%20-
%20Myoglobin%20W.gif
http://themedicalbiochemistrypage.org/images
/myoglobin.jpg
http://web.virginia.edu/Heidi/chapter5/Images
/8883n05_30.jpg