Atomic Structure
advertisement

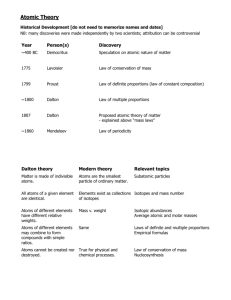
Atomic Structure - HONORS Enduring Understandings: The characteristics of an atom are determined by its structure. The number of protons determines the type of element. Classical physics (mechanics, electricity, and magnetism) lead to the discovery of subatomic particles and the nucleus. Study Guidelines: 1) List the names and symbols of common elements. 2) Describe the historical and present models of the atom. 3) Describe how electrons are arranged in an atom. 4) Identify quarks as particles of matter that make up protons and neutrons. 5) Compute the atomic mass and mass number of an atom. 6) Identify and describe isotopes of common elements. 7) Interpret the average atomic mass of an element. TOTAL POINTS AVAILABLE: 100 (No more than 5 EC points) A 90+ C 70-79 B 80-89 D 60-69 *** Indicates "must do" ***Each student must attempt at least one exploration and application question. Essential level (Minimum 50 points before moving to exploration level) ***Seminar – Discovery log entries are required. 5 pts each Atomic Structure Mass of atoms Reading/Writing 1. Complete any 3 section review questions after reading Chapter 6 and pose 2+ of your own questions about the information you read. 20 pts. 2. Summarize the concepts from the POGIL Atoms and Isotopes worksheet from unit 2 in a paragraph. 5 pts Vocabulary 3. Vocabulary Flash cards for Chapter 6 – in your own words 7 points Worksheets 4. Adopt-an-element 15 points 5. Smaller Particles of Matter (more in-depth than class) 10 points Hands-on activities 6. Make a 3-D model of one of the atoms in periods 2 or 3. Be able to explain both how your model represents an atom and how it is not representative of an atom. 10 points 7. Write an equation to find the average atomic mass using the following variables: X=1st atomic mass, Y=2nd isotope mass, Z=3rd isotope mass, A=% of 1st isotope, B=% of 2nd isotope, C=% of 3rd isotope 7 points ***8. Atomic Jigsaw skit and worksheet 10 points ***9. Nine Fingers Lab 10 points (Replacement assignment: write a paragraph about the elements used to make fireworks and the reasons why we see the energy from an electron dropping orbitals as color) Exploration Level (Choose One or more – 15 Points) 1. Make a display of samples or pictures of several (4 or more different) elements. List the name, symbol, atomic number, average atomic mass, diagram of atom, several uses for each element, and other information you find. 2. Choose a synthetic element and write a biography of the element. Include information about the element’s name, location of the synthesis research, and the people responsible. 3. Research and report on current theorized subatomic particle, such as quarks, leptons, and gluons. (You may make a poster or display of the information.) 4. Make a web page, PowerPoint presentation or 3-D bulletin board to teach others about the history of atom theory and atomic structure. 5. Another idea (Approved by Ms. Mohl) Application Level (Choose One or more – 20 Points) 1. Discuss the benefits and drawbacks of using nanotechnology. 2. Argue whether we should create new atoms. 3. Unravel how color is emitted and how humans detect color. 4. Connect the energy level, classification/name/color, and chemistry of 2+ stars in one page. 5. Another question (Approved by Ms. Mohl)