the additional slides as PowerPoint file.
advertisement
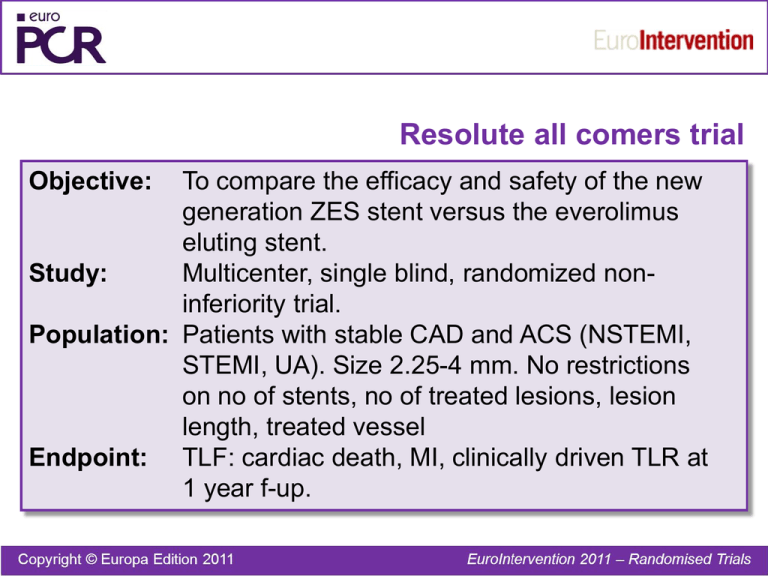
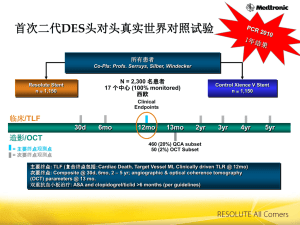
Resolute all comers trial Objective: To compare the efficacy and safety of the new generation ZES stent versus the everolimus eluting stent. Study: Multicenter, single blind, randomized noninferiority trial. Population: Patients with stable CAD and ACS (NSTEMI, STEMI, UA). Size 2.25-4 mm. No restrictions on no of stents, no of treated lesions, lesion length, treated vessel Endpoint: TLF: cardiac death, MI, clinically driven TLR at 1 year f-up. Resolute all comers trial Patients enrolled N=2292 Zotarolimus DES (EndeavorResolute) N=1140 Planned f-up angio 13 m. 20% of patients Everolimus DES (Xience V) N=1152 Planned f-up angio 13 m. 20% of patients Resolute all comers trial Event rate at 1 year f-up (%) p=0.94 p=0.08 p=0.92 p=0.50 zotarolimus 10 p=0.01 everolimus 8,3 8,2 5 1,6 2,8 4,2 4,1 3,9 3,4 1,2 0,3 0 TLF death MI TLR Stent thromb Resolute all comers trial QCA at 13 months f-up angio 30 0,5 21,65 20 Late loss (mm) 19,76 10 0,27 5,2 6,5 0 0,19 0,15 0,06 0,0 in-stent % DS binary restenosis in segment in-stent Resolute all comers trial Conclusion: At follow-up the new generation ZES (Endeavor-Resolute) was found to be noninferior to the everolimus eluting Xience V stent in a population of patients with minimal exclusion criteria. Serruys et al. N Engl J Med 2010;363:136-46