RESOLUTE US
advertisement

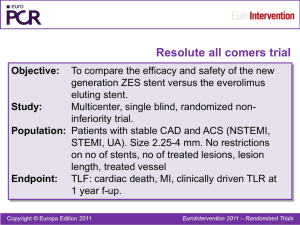
2 Year Clinical Outcomes from the Pivotal RESOLUTE US Study Laura Mauri MD, MSc on behalf of the RESOLUTE US Investigators Brigham and Women’s Hospital Boston, MA ACC 2012 RESOLUTE Global Clinical Program Enrollment Complete - In Follow Up RESOLUTE1 Non-RCT First-in-Human (R=139) 5 yr 1:1 RCT vs. Xience V (R=1140; X=1152) 2 yr RESOLUTE Int4 Non-RCT Observational (R=2349) 2 yr RESOLUTE US5 2.25 – 4.0 mm Non-RCT vs. Hx Control (R=1402) 2 yr 2.5 – 3.5 mm Non-RCT (R=100) vs. Hx Control 2 yr RESOLUTE AC2,3 RESOLUTE Japan R Japan SVS 2.25 Non-RCT vs. PG (R=65) < 1yr 38 mm sub-study Non-RCT vs. PG (R=114) < 1yr 1:1 RCT vs. Taxus (R=200; T=200) < 1yr RESOLUTE Asia Non-RCT Observational (R=312) < 1yr R-China Registry Non-RCT Observational (R=1800) < 1yr Post-approval study (R=230) plan RESOLUTE US R-China RCT Enrolling / Planning RI-US Registry 1 Meredith IT, et al. EuroIntervention. 2010;5:692-7. 2 Serruys PW, et al. N Engl J Med. 2010;363:136-46. 3 Silber S, et al. Lancet. 2011;377:1241-47. 4 Neumann FJ, et al. EuroIntervention. 2012;7(10):1181-8. 5 Yeung AC, et al. JACC. 2011;57:1778-83. RESOLUTE US Clinical Study Design PI: M. Leon, L. Mauri, A. Yeung De Novo Native Coronary Lesion Vessel Diameter: 2.25 – 4.2 mm Lesion Length: ≤ 27 mm (≤ 35 mm lesions tx w/ 38 mm stent) Resolute stent 2.25–3.5 Clinical (n=1242) 2.25–3.5 Angio/IVUS (n=100) 4.0 Angio (n=60) 38mm Clinical (n=110–175) Hx Controls Performance Goals N = max 1577 patients Up to 135 US sites Clinical endpoints 30d 6mo 8mo 9mo 12mo 18mo 2yr Angio/IVUS endpoints Primary Endpoints: • • • • 2.25–3.5 Clinical → Target Lesion Failure at 12mo 2.25–3.5 Angio/IVUS → In-Stent LLL at 8mo 4.0 Angio → In-Segment LLL at 8mo 38 mm Clinical → Target Lesion Failure at 12mo Drug Therapy: ASA and clopidogrel/ticlopidine ≥ 6mo (per guidelines) Mauri L, et al. Am Heart J. 2011;161:807-14. 3yr 4yr 5yr RESOLUTE US Key Eligibility Criteria Key Inclusion Criteria Clinical evidence of ischemic coronary disease Single or double de novo lesion in native coronary artery Target lesion ≤27mm in length, diameter stenosis ≥50% <100%, and target RVD ≥2.25mm ≤4.2mm Target vessel TIMI flow ≥2 Patient is able to take DAPT for at least 6 months Treatment of up to two lesions, if the lesions are located in separate target vessels Mauri L, et al. Am Heart J. 2011;161:807-14. Key Exclusion Criteria Acute MI within 72 hours of index procedure Previous PCI of target vessel within 9 months pre-procedure Planned PCI of any vessel within 30 days post-procedure Hx stroke or TIA within 6 months LVEF <30% RESOLUTE US Patient Flow Chart Patients Enrolled N = 1402 1Yr Clinical Follow-up n = 1386 98.9% 2Yr Clinical Follow-up n = 1359 96.9% RESOLUTE US Baseline Characteristics All Patients (2.25 mm – 4.00 mm diameter) N = 1402 Patients / 1573 Lesions % Age, years (mean ± SD) 64.1±10.7 Male 68.3 Diabetes mellitus 34.4 IDDM 9.6 Prior PCI 32.7 Reason for revascularization: Stable angina 56.1 Unstable angina 41.9 Myocardial infarction 2.1 LAD RVD (mm) 45.9 2.6 ± 0.5 Type B2/C lesion 75.2 Two vessel treatment 10.4 Stents per patient (mean ± SD) Stent length per patient (mm) Yeung AC, et al. J Am Coll Cardiol. 2011;57:1778-83. 1.2 ± 0.5 22.4 ± 10.5 RESOLUTE US – All Patients Target Lesion Failure to 24 Months 25 Cumulative Incidence of TLF (%) RESOLUTE US (N=1402) RESOLUTE All Comers (N=1140) 20 RESOLUTE International (N=2349) 15 11.2% 9.8% 10 7.3% 5 0 0 90 180 270 360 450 Time After Initial Procedure (Days) Error bars indicate a point-wise two-sided 95% confidence interval (±1.96*SE). Standard Error based on the Greenwood Formula. 540 630 720 RESOLUTE US – All Patients Safety and Efficacy Outcomes at 24 Months Events [%] Resolute ZES (n=1359/1402) 99/1359 58/1359 20/1359 26/1359 3/1359 TLF TLR Cardiac Death TV-MI ST (ARC Def/Prob) Target lesion failure (TLF) is defined as cardiac death, target vessel MI and TLR. TLR is ischemia driven. RESOLUTE US – All Patients Clinical Outcomes at 12 and 24 Months 12M 24M n = 1386 n = 1359 1.3 3.0 Cardiac 0.6 1.5 MI (target vessel) 1.3 1.9 Q Wave 0.1 0.1 Non Q wave 1.2 1.8 Cardiac death + target vessel MI 1.9 3.2 ST Def/Prob (all) 0.1 0.2 Early (0-30 days) 0.1 0.1 Late (31-360 days) 0.1 0.1 -- 0.1 TLR 2.8 4.3 TVR 4.5 7.6 TLF (cardiac death, TV-MI, TLR) 4.6 7.3 TVF 6.1 10.1 % Death (all) Very late (>360 days) DAPT to 24 Months Adherence to DAPT (%) RESOLUTE US, All Comers, International Trials 100 97.3 97.1 95.8 95.5 90 93.8 93.1 80 R-US (N=1402) R-INT (N=2349) R-AC (N=1140) 91.1 93.5 84.1 73.8 70 67.2% 60 50 43.9% 40 30 20 18.6% 10 0 30 180 360 Time After Initial Procedure (days) 540 720 Def/prob Stent Thrombosis to 24 Months RESOLUTE US, All Comers, International Trials 25 RESOLUTE US (N=1402) RESOLUTE All Comers (N=1140) 20 RESOLUTE International (N=2349) 15 10 5 0 0 90 180 270 360 450 540 Time After Initial Procedure (days) Error bars indicate a point-wise two-sided 95% confidence interval (±1.96*SE). Standard Error based on the Greenwood Formula. 630 1.9% 1.0% 0.2% 720 RESOLUTE US – All Patients Stent Thrombosis at 24 Months 30 Days n=1399 1 Year n=1386 2 Year n=1359 0.1 (1) 0.1 (2) 0.2 (3) 0.1 (1) 0.1 (1) 0.1 (1) Late (30-360 days) n/a 0.1 (1) 0.1 (1) Very Late (>360 days) n/a n/a 0.1 (1) % (n) Stent thrombosis (ARC def/prob) Early (< 30 days) RESOLUTE US Main Study (2.5-3.5mm single lesion) Safety and Efficacy Outcomes at 24 Months Events [%] Resolute ZES (n=968/1001) 57/968 TLF 10/968 Cardiac Death 17/968 33/968 1/968 TV-MI TLR ST (ARC Def/Prob) Target lesion failure (TLF) is defined as cardiac death, target vessel MI and TLR (ischemia driven). TLF rate at 2 years for Endeavor ZES was 8.1% (Post-hoc analysis, Pnoninferiority<0.001) RESOLUTE US – 2.25mm Cohort Safety and Efficacy Outcomes at 24 Months Events [%] Resolute ZES (n=147/150) 12/147 TLF 5/147 Cardiac Death 2/147 TV-MI Target lesion failure (TLF) is defined as cardiac death, target vessel MI and TLR. TLR is ischemia driven. 7/147 TLR 2/147 ST (ARC Def/Prob) RESOLUTE US – Diabetic Patients Safety and Efficacy Outcomes at 24 Months Events [%] Resolute ZES (n=474/482) 42/474 TLF 10/474 Cardiac Death 7/474 27/474 0/474 TV-MI TLR ST (ARC Def/Prob) Target lesion failure (TLF) is defined as cardiac death, target vessel MI and TLR. TLR is ischemia driven. RESOLUTE US – Diabetic Patients Safety and Efficacy Outcomes at 24 Months Diabetic (n=474/482) Events [%] Non-Diabetic (n=885/920) 42/474 57/885 TLF 10/474 10/885 Cardiac Death 7/474 19/885 TV-MI Target lesion failure (TLF) is defined as cardiac death, target vessel MI and TLR. TLR is ischemia driven. 27/474 31/885 TLR 0/474 3/885 ST (ARC Def/Prob) RESOLUTE US Conclusions • Out to 2 years, the TLF (primary endpoint) was consistently low in all groups studied – All patients 7.3%, main cohort (2.5-3.5 single lesion) 5.9%, small vessels (2.25 mm) 8.2% • Stent thrombosis was very low (0.2%) with only 1 new ST event after 1 year, indicating a favorable safety profile • Very good outcomes in the diabetic subgroup – Low rate of TLF (8.9%) and TLR (5.7%) – No stent thrombosis out to 2 years