Partner TM
advertisement

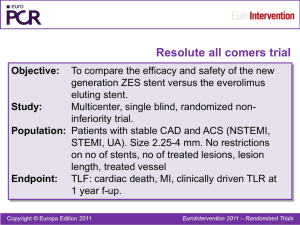
Partner Durable Polymer and NANO Polymer-Free Sirolimus-Eluting Stents: Update on Clinical Data Prof. Ben He MD PhD FACC Renji Hospital, Shanghai Jiaotong Univ Components of First Generation DES Drug Polymer Stent • • • SMC inhibition Pro-endothelial growing Inflamation inhibition •Carrier of Drug, •Control release • Plaque compression • Vessel scaffolding • Delievery System helper for stent delievery ® Polymer-durable Sirolimus-Eluting Stents Partner 多聚物载体雷帕霉素药物洗脱支架 PBMA/PEVA聚合物,双涂层 能有效、安全地预防再狭窄 ® Partner DES Polymer-durable ——Retrospective Clinical Data Organizer & Administrator Purpose:To evaluate the efficacy and safety of Partner®DES in Chinese patients。 Prof. Yong Huo Clinical results in 12 month n=1352 5.0 3.62 4.0 2.14 %3.0 1.63 2.0 1.0 0.52 0.96 0.22 0.0 Cardiac NonDeath cardiac Death MI TLR TVR MACE* *MACE: cardiac death、MI、TVR Clinical results in 24 month n=1352 10.0 6.58 8.0 % 3.70 6.0 2.96 4.0 2.0 2.07 0.81 0.37 0.0 Cardiac NonDeath cardiac Death MI TLR TVR MACE* *MACE: cardiac death、MI、TVR Compare with other data Partner Retrospective n=1352 SIRIUS1 n=533 TAXUS IV2 n=662 DES Partner Cypher Taxus Lesion lenth, mm 21.99±10.57 14.4±5.7 13.4±6.3 DM, % 23.30 24.6 23.4 1yr MACEs rate, % 3.62 8.3 10.6 1yr cardiac death rate, % 0.52 1.3 1.4 1yr MI rate, % 0.96 3.0 3.5 1yr TLR rate, % 1.63 4.9 4.2 2yrs MACEs rate,% 6.58 10.9 14.7 2yrs TLR rate,% 2.96 6.3 5.6 1. David R. Holmes et al., Circulation 2004;109;634-640 2. Gregg W. Stone et al., Circulation (2004;109:1942-1947). Conclusions from Respective data on 1st Generation of Partner Partner® Stent Retrospective study reflect the real world daily practice in China Partner®Stent is an effective and safety stent Partner®Stent is comparable to the current daily practice imported stents 1st Generation of DES, Success but far from Perfect Mechanisms for Late stent Thrombosis NanoTM Polymer-Free Sirolimus-Eluting Stents 无多聚物载体的雷帕霉素洗脱支架 支架表面纳米级微孔作为药物载体 由于没有多聚物载体,更少免疫反应、从而更 少血栓、更安全 与裸金属支架相同的安全性,与药物支架相同 的有效性 Smooth in stent surface 载药纳米微孔 载药后药物均匀分布 NanoTM无载体支架载药量: 2.2μg/mm2 Late lumen loss decreased significantly BMS Polymer SES Polymer-free SES 1个月组织学和造影 动物试验结果表明: 无载体SES的管腔丢失较 裸支架显著降低 与多聚物载体SES相似 QCA Neointimal area decreased significantly 1个月IVUS动物试验显示:新生内膜面积与BMS相比显著降低 与多聚物载体SES相比无明显差异 Total endotheliazation after 1 month B A A length 0.34mm B length 0.11mm OCT观察动物血管内皮愈合情况显示,植入后一个月,内皮已完全愈合 Less stent related inflamation ..\..\临床研究资料\Paper1\fig5\FIG8.jpg BMS Polymer SES Polymer-free SES 6个月组织学动物试验结果表明: 无载体支架的免疫反应较 多聚物载体支架显著降低 与裸支架相似 Pre-Clinical results : Summary 0.163 0.5 1.833 0.833 Low inflamation Total endothelialization Similar late loss to DES Similar thrombogenecity to BMS BMS-like safety DES ® Partner Polymer-Free SirolimusEluting Stents: N-FIM Purpose To evaluate the safety and efficacy of NanoTMin Clinical 组织和管理 主要研究单位:北京大学第一医院 主要研究者:霍勇 核心实验室:徐波,介入导管室,阜外心血管病 医院 数据管理及统计处理:北京大学第一医院统计室 参与中心及主要研究者 北京大学第一医院 霍勇 辽宁省人民医院 李占全 北京安贞医院 陈方 第四军医大学西京医院 王海昌 广东省人民医院 陈纪言 北京大学人民医院 王伟民 复旦大学附属中山医院 葛均波 北京医院 何青 北京朝阳医院 王乐丰 吉林大学第二医院 李淑梅 中国医科大学附属第一医院 齐国先 江苏省人民医院 杨志健 上海仁济医院 何奔 北京宣武医院 李康 北京同仁医院 李田昌 华中科技大学附属同济医院 曾和松 北京世纪坛医院 彭建军 武警医学院附属医院 姜铁民 华中科技大学附属协和医院 曾秋棠 浙江大学医学院附属第一医院 朱建华 浙江大学医学院附属邵逸夫医院 傅国胜 Population Including criteria Target vessel ref dia.2.5-4.0mm Target lesion stenosis≥70% Lesion length coverable for 1 stent lenth≤40mm Patient agreement for Clin& Angio f/u Inform consent Excluding creteria AMI< 1wk Study Design Prospective ,Multicenters, Controlled Trial V diameter 2.5-4.0mm Lesion stenosis≥70% Lesion length coverabled by 1 stent with≤40mm length N=200 Poly free NanoTMSES 1:1 Partner PolymerTMSES Clinical F/U:1,3,6,12 month CAG F/U:6个月 Clopidegrel for 6m,ASP Lifelong Study Endpoints Primary endpoints - In stent & In segment late lumen loss at 6 month Secondary endpoints 6 month MACE: death,MI,TVR, 12 month MACE: death,MI,TVR, Inclusion & Follow-up Stent Pt number 6M Clin f/u 6-8M CAG f/u 12M Clin f/u NanoTM 143 143 (100%) 95 (66.4%) 143 (100%) Partner 147 147(100%) 94 (63.9%) 147(100%) TM Baseline Demographic NanoTM 患者 age (岁) n=143 56.92±10.42 PartnerTM P n=147 59.47 ±9.82 0.56 male 76.04% 79.22% 0.62 Previous MI 34.38% 23.38% 0.11 DM 15.63% 18.18% 0.65 Hypertension 58.33% 50.65% 0.31 Hyperlipidemia 27.08% 35.06% 0.26 CAD family history 4.17% 2.60% 0.58 Current smoker 55.21% 49.35% 0.44 ACC/AHA Lesion types TM Nano 试验组 A B1 B2 C TM Partner 对照组 A B1 B2 C Pre-intervention Angiographic results Nano Partner 179 189 Baseline, n Mean SD Mean SD P Pre-procedure reference vessel diameter (mm) 2.79 0.47 2.81 0.52 0.59 Pre-procedure diameter stenosis (%) 73.21 14.69 69.04 14.39 0.006 Pre-procedure minimal lumen diameter (mm) 0.76 0.46 0.88 0.45 0.01 Pre-procedure lesion length (mm) 19.58 12.44 20.57 12.04 0.44 Results post intervention NanoTM PartnerTM P Post-procedure reference vessel diameter (mm) 3.12 0.50 3.17 0.52 0.30 Post-procedure in-stent minimal lumen diameter (mm) 2.67 0.48 2.71 0.49 0.38 Post-procedure prox-edge minimal lumen diameter (mm) 3.02 0.63 3.09 0.64 0.28 Post-procedure dist-edge minimal lumen diameter (mm) 2.49 0.64 2.51 0.65 0.70 Post-procedure in-segment minimal lumen diameter (mm) 2.40 0.56 2.44 0.57 0.647 Acute gain in-stent (mm) 1.91 0.51 1.83 0.52 0.17 Acute gain in-segment (mm) 1.63 0.56 1.56 0.56 0.20 CAG follou/up results at 6-8m Follow-up, n NanoTM PartnerTM 112 123 Mean SD Mean SD P 3.04 0.49 3.07 0.50 0.69 2.33 0.60 2.43 0.58 0.17 2.77 0.68 2.76 0.68 0.91 2.36 0.64 2.27 0.61 0.28 Follow-up in-segment minimal lumen diameter (mm) 2.11 0.66 2.13 0.58 0.81 Late loss in-stent (mm) 0.37 0.40 0.26 0.40 0.03 Late loss prox-edge (mm) 0.28 0.45 0.31 0.55 0.58 Late loss dist-edge (mm) 0.20 0.26 0.22 0.44 0.61 Late loss in-segment (mm) 0.34 0.42 0.30 0.48 0.54 Follow-up reference vessel diameter (mm) Follow-up in-stent minimal lumen diameter (mm) Follow-up prox-edge minimal lumen diameter (mm) Follow-up dist-edge minimal lumen diameter (mm) MLD in 6-8 months f/u Late loss in 6-8 months f/u— In stent Late loss in 6-8 months f/u— In Lesion Late loss in 6-8 months f/u— In Lesion 0.45 P=0.54 0.34 0.30 0.3 0.15 In-lesion Late loss (mm) 1 2 NanoTM PartnerTM Distribution of late loss in 68months CAG f/u 0.4 P=0.03 P=0.58 0.37 0.31 0.3 0.26 0.28 P=0.61 0.20 0.2 0.22 0.1 0 Late loss in-stent (mm) NanoTM PartnerTM Late loss prox-edge (mm) Late loss dist-edge (mm) In stent restenosis in 6-8 months CAG f/u P=0.63 6.00% 4.46% 4.00% 3.25% 2.00% 0.00% 1 2 In-stent Binary Restenosis Rate NanoTM PartnerTM Main clinical results in 6-8 months 5.00% 4.00% 3.00% 2.00% 1.00% 0.00% 4.20% 4.20% 3.40% 3.40% NanoTM PartnerTM TVR(%) MACE (%) NanoTM (n=143) PartnerTM (n=147) Death 0 0 MI 1 0 TLR 6 3 TVR 6 5 Main clinical results in 12 months P=0.69 P=0.69 6.00% 5.00% 4.00% 3.00% 2.00% 1.00% 0.00% 4.90% 4.90% 4.08% 4.08% NanoTM PartnerTM TVR(%) MACE (%) NanoTM (n=143) PartnerTM (n=147) Death 0 0 MI 1 0 TLR 7 4 TVR 7 6 无晚期血栓形成 N-FIM Compare with others SIURIUS TAXUS IV ENDEAVOR II SPIRIT III N-FIM 药物支架,n Cypher Taxus Endeavor Xience V Nano 病例数,n 533 662 598 223 121 参考管腔直径, mm 2.79 2.75 2.74 2.70 2.78 病变长度,mm 14.4 13.4 14.05 13.0 19.58 糖尿病,% 25 23.4 18 23 16.5 6-9个月LL,mm 0.17 0.39 0.61 0.11 0.34 6-9个月再狭窄率, % 8.9 7.9 13.2 3.4 4.46 1年TLR,% 4.9 4.2 4.5 3.4 4.9 1年MACE,% 8.3 10.6 7.6 6.0 4.9 N-FIM Conclusion FIM 1yr clinical f/u completed 6-8 month NanoTM SES effectively prevented restenosis,the LL & Binary restenosis is comparable to PartnerTM 12month clinical f/u showed MACE,death、MI、TVR are similar in this 2 stent types。 Need more data to evaluate NanoTM SES in the prevention of Late stent thrombus Thank you for your attention