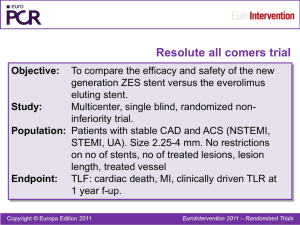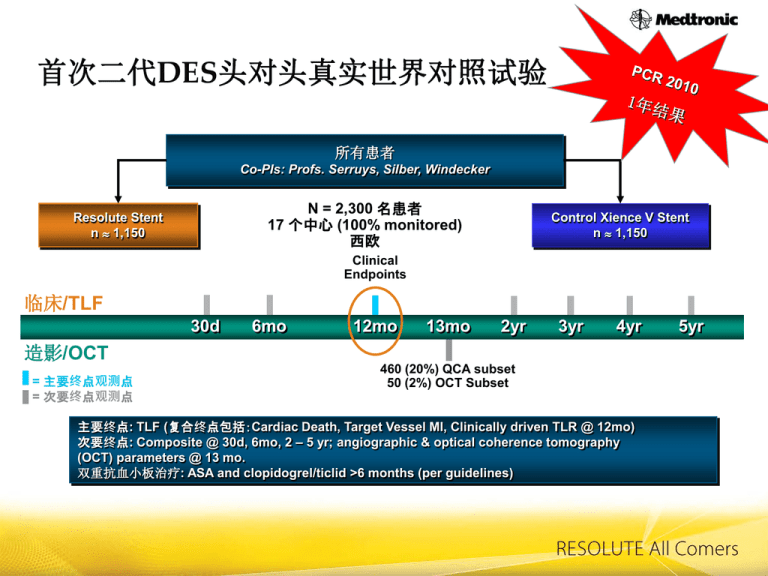
首次二代DES头对头真实世界对照试验
所有患者
Co-PIs: Profs. Serruys, Silber, Windecker
N = 2,300 名患者
17 个中心 (100% monitored)
西欧
Resolute Stent
n 1,150
Control Xience V Stent
n 1,150
Clinical
Endpoints
临床/TLF
30d
6mo
12mo
13mo
2yr
3yr
4yr
造影/OCT
= 主要终点观测点
= 次要终点观测点
460 (20%) QCA subset
50 (2%) OCT Subset
主要终点: TLF (复合终点包括:Cardiac Death, Target Vessel MI, Clinically driven TLR @ 12mo)
次要终点: Composite @ 30d, 6mo, 2 – 5 yr; angiographic & optical coherence tomography
(OCT) parameters @ 13 mo.
双重抗血小板治疗: ASA and clopidogrel/ticlid >6 months (per guidelines)
5yr
Resolute DES
Xience V DES
(n = 1140)
(n = 1152)
p-value
Age (yr)
64.4 ±10.9
64.2 ±10.8
NS
Men (%)
76.7
77.2
NS
Diabetes mellitus (%)
23.5
23.4
NS
ACS (%)
48.3
47.7
NS
AMI (within 12 hr) (%)
15.4
17.8
NS
AMI (within 72 hr) (%)
28.9
28.8
NS
1.46 ±0.73
1.48 ±0.77
NS
Multivessel disease (%)
58.4
59.2
NS
Small vessel (RVD ≤2.75 mm)
67.8
67.4
NS
Long lesion (length >18 mm)
18.2
21.2
NS
In-stent restenosis (%)
8.1
8.0
NS
1.90 ±1.21
2.02 ±1.34
0.02
Bifurcation/trifurcation (%)
16.9
17.7
NS
Total occlusion (%)
16.3
17.2
NS
Complex Patients1 (%)
67.0
65.6
NS
Lesions treated per patient
Stents per patient
1
Complex patient definition: Bifurcation, SVG, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal
insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO
(preprocedure TIMI = 0). With the exception of long lesions (treatable with a single 38-mm length stent), Resolute DES
currently is not specifically approved for the patient subsets noted in this complex patient definition.
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan.
© 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
试验入组充分反映临床实际
Enrollment
Reflects Complex Real-World Practice
Resolute达到主要终点12个月TLF
Resolute
Matches Xience V DES in Primary Endpoint
Target Lesion Failure (TLF)
Cumulative incidence of events (%)
20
Log Rank p = 0.92
Xience V (n = 1152)
15
Resolute (n = 1140)
8.3
10
8.2
5
0
0
180
Time after initial procedure (days)
360
No. Entered
Resolute
0
1140
30
1110
60
1084
90
1076
120
1070
150
1062
180
1060
210
1058
240
1051
270
1042
300
1038
330
1037
360
1025
Xience V
1152
1123
1088
1080
1078
1074
1068
1061
1047
1046
1038
1032
1019
TLF = Cardiac death, target vessel MI, TLR.
Error bars indicate a point-wise two-sided 95% confidence interval (±1.96 * SE).
Standard error based on the Greenwood formula.
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan. © 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
MACE (%)
TVF (%)
(D, MI, eCABG, TLR)
(CD, TV MI, TVR)
9.7
9.6
p = 0.42
p = 0.66
9.0
8.7
Xience V DES
Resolute DES
n = 1126
n = 1119
Xience V DES
p-values are based on Fisher's Exact Test.
p-values for outcome differences are unadjusted for multiple comparisons.
RESOLUTE All Comers was not specifically designed or powered to individually compare MACE and TVF.
n = 1126
Resolute DES
n = 1119
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan.
© 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
Resolute:MACE和TVF发生率
Resolute
DES Shows Numerically Lower MACE
and TVF
Xience V DES
Resolute DES
n = 1126
p = 0.50
p = 0.92
4.8
3.4
n = 1119
p = 0.08
p = 0.61
4.9
4.1
3.9
4.2
2.8
1.6
TLR (%)
p = 0.92
TVR (%)
Death (%)
1.7
1.3
CD (%)
TVMI (%)
靶血管心梗
p-values are based on Fisher's Exact Test.
p-values for outcome differences are unadjusted for multiple comparisons.
RESOLUTE All Comers was not specifically designed or powered to individually compare endpoints shown above.
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan.
© 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
Resolute:各个临床终点的出色表现
Resolute
DES Shows Excellent Performance Across
All Clinical Efficacy and Safety Endpoints
Resolute和Xience V:血栓无统计学差异
• Resolute:56%血栓发生在术后5天内
• 早期的血栓对12个月的CD/TVMI无影响
ST (ARC Def/Prob)
Acute
(0–1 day)
Subacute
(2–30 days)
Late
(31–360 days)
All
Resolute DES (%)
0.4
0.7
0.6
1.6
Xience V DES (%)
0.2
0.4
0.2
0.7
p-value
NS
NS
NS
NS
p-values are based on Fisher's Exact Test.
p-values for outcome differences are unadjusted for multiple comparisons.
Per protocol, a statistical difference was declared if the two-sided p-value was less than 0.05
RESOLUTE All Comers was not specifically designed or powered to individually compare endpoints shown above.
Resolute:早期的血栓对12个月的CD/TVMI无影响
Cardiac Death and TV MI
ARC Definite/Probable ST
10
Xience V (N = 1152)
Cumulative incidence of events [%]
Cumulative incidence of events [%]
10
Resolute (N = 1140)
Log Rank p = 0.05
5
0
Xience V (N = 1152)
Resolute (N = 1140)
Log Rank p = 0.96
5
0
0
180
360
0
Time after initial procedure [days]
早期血栓Resolute略高
180
360
Time after initial procedure [days]
早期CD/TVMI血栓Xience V较高
Error bars indicate a point-wise two-sided 95% confidence interval (±1.96*SE). Standard Error based on the Greenwood Formula
p-values for outcome differences are unadjusted for multiple comparisons.
RESOLUTE All Comers was not specifically designed or powered to individually compare endpoints shown above
In-Stent Diameter Stenosis (%)
Powered secondary endpoint
In-Stent Late Loss and Distribution
In-stent Late Loss:
Resolute 0.27 mm vs. Xience V 0.19 mm (p = 0.08)
pnonInferiority = 0.035
21.7
19.8
Xience V DES
Resolute DES
n = 186
n = 191
Cumulative Frequency (%)
100
p = 0.21
Noninferiority p-values calculated based on a prespecified noninferiority margin of 5%
Other listed p-values are based on t-test and are unadjusted for multiple comparisons..
RESOLUTE All Comers was not specifically designed or powered to compare late loss at 13 months.
75
Resolute DES
Xience V DES
50
25
0
-1.00
0.00
1.00
2.00
In-Stent Late Loss (mm)
3.00
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan.
© 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
Resolute和Xience
V: 13个月DS%和LL无差异
No
Significant Difference
in Diameter Stenosis or
In-Stent Late Loss at 13 months
All Patients
N = 1520/2292
34%
Simple
66%
Complex
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan.
© 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
Resolute All Comers:70%的复杂病患
TLF (%) in Complex Patients
MACE (%) in Complex Patients
p = 0.66
p = 0.15
11.5
20%
9.7
8%
9.2
8.9
Xience V DES
Resolute DES
Xience V DES
Resolute DES
n = 742
n = 752
n = 742
n = 752
Complex patient definition: Bifurcation, SVG, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal insufficiency or failure
(creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO (preprocedure TIMI = 0). With the exception
of long lesions (treatable with a single 38-mm length stent), Resolute DES currently is not specifically approved for the patient subsets noted in
this complex patient definition. p-values are based on Fisher's Exact Test. p-values for outcome differences are unadjusted for multiple
comparisons. RESOLUTE All Comers was not specifically designed or powered for complex patient subset analysis.
For distribution only in markets where Resolute DES has been approved. Not for distribution in the USA or Japan.
© 2010 Medtronic, Inc. All rights reserved. UC201006397EE 5/10
Resolute:在复杂病变中事件率
Resolute
DES Shows Strong Performance in
Complex Patients
RESOLUTE All Comers 和 LEADERS
相同的试验设计
RESOLUTE All Comers
LEADERS
Co-PIs: Profs. Serruys, Silber, Windecker
PI: Prof. Windecker
Real World
All Comers with symptomatic
coronary artery disease
Stable and ACS Patients
undergoing PCI
N = 2,300 patients
17 sites (100% monitored)
Western Europe
Resolute Stent
n 1,150
N = 1,700 patients
10 sites (100% monitored)
Western Europe
BioMatrix Flex
n 850
Xience V Stent
n 1,150
Clinical/TLF
Cypher Select
n 850
Clinical
30d
6mo 12mo 13mo 2yr
3yr
4yr
5yr
Angio/OCT
30d
Angio/OCT
460 (20%) QCA subset
50 (2%) OCT subset
1º Endpoint: TLF – Cardiac Death, TV-MI, clinically driven TLR @ 12-mo
2º Endpoints: Composite @ 30d, 6mo, 2 – 5 yr; angiographic & optical
coherence tomography (OCT) parameters @ 13 mo
Drug Therapy: ASA and clopidogrel/ticlid >6 months (per guidelines)
6mo 9mo
12mo
2yr
3yr
4yr
420 (25%) QCA subset
46 (3%) OCT subset
1º Endpoint: Cardiac Death, MI, clinically-driven TVR @ 9-mo
2º Endpoints: Death, Cardiac Death, MI, TLR, TVR, ARC ST
Drug Therapy: ASA and clopidogrel/ticlid recommended
12 months
5yr
RESOLUTE All Comers 和 LEADERS 试验
RESOLUTE All
Comers
Resolute DES
N = 1140
RESOLUTE All
Comers
Xience V DES
N = 1152
LEADERS
BioMatrix Flex
DES
N = 857
LEADERS
Cypher
Select DES
N = 850
Age (yr)
64.4 ±10.9
64.2 ±10.8
65 11
65 11
Men (%)
76.7
77.2
75
75
Diabetes mellitus (%)
23.5
23.4
26
23
ACS (%)
48.3
47.7
55%
56%
Unstable Angina (%)
19.4
18.9
22%
20%
Lesions treated per patient
1.46 ±0.73
1.48 ±0.77
1.5 0.7
1.4 0.7
Prior myocardial infarction
28.9%
30.4%
32%
33%
Prior PCI
31.8%
32.1%
36%
37%
67.8
67.4
68%
69%
Small vessel (RVD ≤2.75
mm)
12个月真实世界所有患者的对比:第一代 Vs.第二代
TVF (%)
BioMatrix Flex DES (n=857)
Xience V DES (n=1126)
Cypher Select DES (n=850)
Resolute DES (n=1119)
CD, MI, ID-TVR
12.1
10.7
ST (%)
ID-TLR (%)
9.6
ARC Def/Prob
9.0
5.1
5.8
3.4
3.9
2.8
2.2
0.7
LEADERS
RESOLUTE
All Comers
LEADERS
RESOLUTE
All Comers
Data come from different trials and could differ in a head-to-head comparison.
LEADERS
1.6
RESOLUTE
All Comers