Writing Empirical and Molecular Formulas
Writing Empirical and
Molecular Formulas
What is an empirical formula?
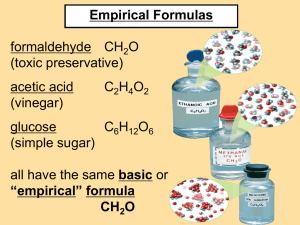
A chemical formula in which the ratio of the elements are in the lowest terms is called an empirical formula .
Example:
The empirical formula for a glucose molecule (C
6
H
12
O
6
) is CH
2
O. All the subscripts are divisible by six.
C
6
H
12
O
6 6 6
6
C H
2
O
Exceptions:
Some formulas, such as the one for carbon dioxide, CO
2
, are already empirical formulas without being reduced .
Calculating empirical formula:
To find the empirical formula from the molecular formula, you must divide all the subscripts by the (GCF) greatest common factor .
Determine the empirical formulas for each of the following molecular formulas.
1. C
8
4. C
3
H
18
2. H
2
O
2
3. Hg
2
H
…..______________
……______________
Cl
2
6
…..______________
…….______________
5. Na
2
C
2
O
4
..._____________
6. H
2
O......________________
7. C
4
H
8
….._______________
8. C
9. C
4
7
H
H
10. CH
6
…..________________
12
…._______________
3
COOH….___________
Finding empirical formulas using molecular mass:
Find mass (or %) of each element.
Find moles of each element.
Divide moles by the smallest # to find subscripts.
When necessary, multiply subscripts by
2, 3, or 4 to get whole #’s.
EXAMPLE: Find the empirical formula for a sample of 25.9% N and 74.1% O.
25.9g = 1.85 mol N 1.85mol N = 1 N x 2 = N
2
14g/mol 1.85 mol
74.1 g = 4.63 mol O 4.63 mol O = 2.5 O x 2 = O
5
16 g/mol 1.85 mol
N
2
O
5
PRACTICE PROBLEMS:
1.
A substance is 36.1% by weight calcium and 63.9% chlorine. What is the empirical formula of this compound?
2. A compound is 43.4%Na, 11.3%C, and
45.3% O. What is the empirical formula for this compound?
3. A compound is 2.46%H, 39.1%S, and
58.5%O. What is the empirical formula for this compound?
What is a molecular formula?
A molecular formula is the “ true formula ” of a compound. The chemical formula for a molecular compound shows the actual number of atoms present in a molecule .
To find the molecular formula from the empirical formula:
Find the empirical formula.
Determine the empirical formula mass.
Divide the molecular mass by the empirical formula mass to determine the multiple.
Multiply the empirical formula by the multiple to find the molecular formula.
MF mass = n
EF mass
(EF)n = molecular formula
EXAMPLE:
The empirical formula for ethylene is CH
2.
molecular formula if the molecular mass is
Find the
28.1g/mol.
C = 1 x 12 = 12
H = 2 x 1 = +2
14g/mol = empirical formula mass
28.1 g/mol = 2
14 g/mol
(CH
2
)
2
C
2
H
4
Practice Problems:
1. Find the molecular formula for a compound with a mass of 78 amu and the empirical formula CH.
2. Find the molecular formula for a compound with a mass of 82 amu and the empirical formula C
3
H
5
.
3. Find the molecular formula for a compound with a mass of 90 amu and the empirical formula HCO
2
.
4. Find the molecular formula for a compound with a mass of 112 amu and the empirical formula CH
2
.
5. Find the molecular formula for a compound with a mass of 40 amu and the empirical formula C
3
H
4
.
More Practice Problems:
Writing Empirical Formulas:
1.Determine the empirical formula of a compound containing 2.644 g of gold and
0.476 of chlorine.
2. Determine the empirical formula of a compound containing 0.928 g of gallium and 0.412 of phosphorus.
Writing Molecular Formulas:
9.
Find the molecular formula of a compound that contains 42.56g of palladium and 0.80g of hydrogen. The molar mass of the compound is 216. g/mol.