Empirical Formula - Prairie Spirit Blogs
advertisement

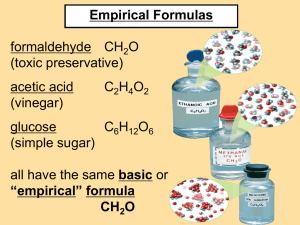
4.5 EMPIRICAL FORMULAS Formulas • Law of Constant Composition - a compound contains elements in a certain fixed proportions (ratios), regardless of how the compound is prepared or where it is found in nature. • If you have one molecule of methane gas, you will always have 1 carbon atoms and 4 hydrogen atoms. 3 3 Steps for determining Chemical Formulas 1. Determine the percent composition of all elements. 2. Convert this information into an empirical formula 3. Find the true number of atoms/ elements in the compound (Molecular Formula) 4.5 Empirical Formula 2. Empirical Formula is the formula that gives the lowest ratio of atoms in a compound. It does not necessarily tell you the exact number of each type of atom. Steps to Determine Empirical Formula 1. List given values. 2. Write the mass percent as mass (mass percent is just mass per 100g sample). 3. Convert each mass to moles. 4. State the mole ratios. 5. Calculate lowest whole number ratio. Example 1: The percent composition of a compound is 69.9 % iron and 30.1% oxygen. What is the empirical formula of a compound? Step 1: List the given values Fe = 69.9% and O = 30.1% Step 2: Calculate the mass (m) of each element in a 100g sample. mFe= 69.9 x 100g = 69.9g 100 mO= 30.1 x 100g = 30.1g 100 Step 3: Convert Mass (m) into moles (n) nFe= 69.9g x 1mol/ 55.86g = 1.25 mol Fe nO= 30.1g x 1mol/ 16.00g = 1.88 mol O Step 4: State the Amount Ratio nFe : nO 1.25mol : 1.88 mol Step 5: Calculate lowest whole number ratio 1.25mol : 1.88 mol 1.25mol 1.25 mol 1 : 1.5 2 : 3 When you don’t get a whole number, multiply entire ratio by 2, 3, 4 etc. until you get a whole number Empirical Formula is Fe2O3 Example 2: The percentage composition of a compound is 21.6% sodium, 33.3% chlorine, and 45.1% oxygen. What is the empirical formula of the compound? Step 1: List the given values Cl=33.3%, Na = 21.6% and O = 45.1% Step 2: Calculate the mass (m) of each element in a 100g sample. mNa= 21.6 x 100g = 21.6g Na 100 mCl= 33.3 x 100g = 33.3g Cl 100 mO= 45.1 x 100g = 45.1g O 100 Step 3: Convert Mass (m) into moles (n) nNa= 21.6g x 1mol/ 23.0g = 0.94 mol Na nCl= 33.3g x 1mol/ 35.5g = 0.94 mol Cl nO= 45.1g x 1mol/ 16.00g = 2.82 mol O Step 4: State the Amount Ratio nNa : nCl : nO 0.94mol : 0.94mol : 2.82 mol Step 5: Calculate lowest whole number ratio 0.94mol : 0.94mol : 2.82 mol 0.94mol : 0.94mol : 0.94 mol 1 : 1: 3 Empirical Formula is NaClO3 Example 3: A barium salt is found to contain 21.93 g of barium, 5.12 g of sulfur, and 10.24 g of oxygen. What is the simplest formula of this compound? Step 1: List the given values Ba = 21.93g S = 5.12g O = 10.24g Step 2: Calculate the mass (m) of each element. ***Mass is given, so proceed to step 3! Assignment • Proceed with the assignment that follows Empirical Formula Check Point 1. Determine the empirical formula for the following compound: 22.1% aluminum, 25.4 % phosphorus and 52.5% oxygen 2. Name the compound! ;)