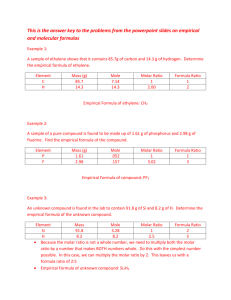
empirical formula
advertisement
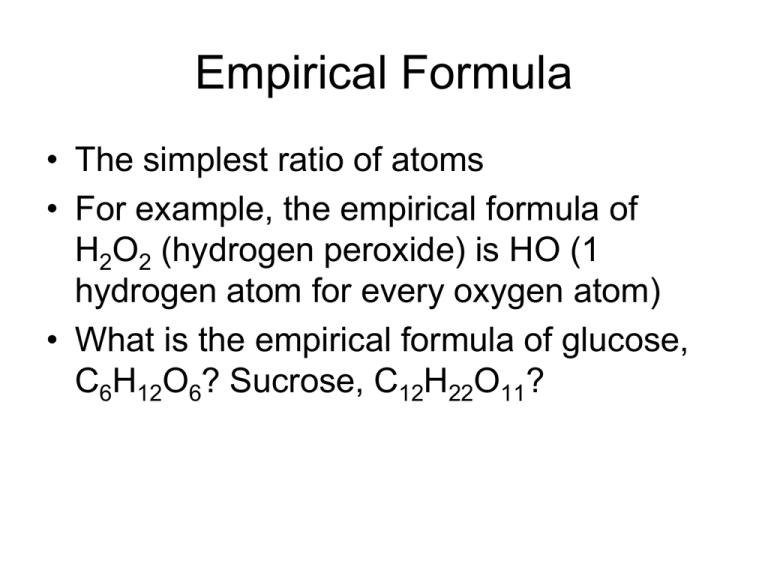
Empirical Formula • The simplest ratio of atoms • For example, the empirical formula of H2O2 (hydrogen peroxide) is HO (1 hydrogen atom for every oxygen atom) • What is the empirical formula of glucose, C6H12O6? Sucrose, C12H22O11? How to calculate EF Q: Calculate the EF of a compound containing 49.5% C, 5.2% H, 28.8% N and 16.5 % O by mass. 1.Arrange the elements in % or mass 49.5g C, 5.2g H, 28.8g N, 16.5g O 2.Divide each by the molar mass of the element to get moles C49.5g H5.2g N28.8g O16.5g 12gmol-1 1 gmol-1 14gmol-1 16gmol-1 C 4.125 H 5.20 N mol mol 2.056 mol O 1.031 mol 4. Divide everything by the SMALLEST NUMBER of moles 4.125 5.2 2.056 1.031 1.031 1.031 1.031 1.031 = 4 : 5 : 2 : 1 5. Write the formula with the simplest whole number ratio C4H5N2O ANSWER Now try these! Calculate the EF: 1. Nutrasweet is 57.14% C, 6.16% H, 9.52% N and 27.18% O. 2. A compound is 72.2% Mg and 27.8% N 3. An oxide of nitrogen contains 42.05 g N and 95.95 g of O. 4. Mercury forms a compound that is 73.9% Hg and 26.1% Cl. 5. Vitamin C contains 40.92% C, 4.58% H and 54.50% O. Now try these - ANSWERS 1. 2. 3. 4. 5. C14H18N2O5 Mg3N2 NO2 HgCl2 C 3H 4O 3 From EF to molecular formula • The molecular formula is the ACTUAL NUMBER OF EACH ATOM in the compound HO Multiplication factor x2 H2O2 (hydrogen peroxide) CH2O CH2O X6 x2 C6H12O6 (glucose) CH3COOH (ethanoic acid) EF MF How to work out the MF 1. Calculate the molar mass for the empirical formula. Example: for a compound where the EF is HO and actual molar mass is 34 gmol-1 Molar mass of EF = 1 + 16 = 17 gmol-1 2. Divide the molar mass MF ÷ EF Actual MF ÷ EF 34 ÷ 17 gmol-1 = 2 3. Multiply the formula by the factor calculated in part 2. HO x 2 = H2O2 Questions – Molecular formula 1. A compound has an empirical formula of CH2Cl and a molar mass of 99 gmol-1. Calculate the molecular formula. 2. A compound has 75.46% C, 4.43% H and 20.1% O and a molar mass of 318 gmol-1. Calculate the molecular formula. Answers – Molecular formula 1. The EF is CH2Cl. The molar mass for this is 12 + 2 x 1 + 35.5 = 49.5. 99 ÷ 49.5 = 2, therefore the molecular formula is TWICE that of the EF – C2H4Cl2. 2. The EF is C10H7O2. The molar mass for this is 12 x 10 + 7 x 1 + 2 x 16 = 159. 318 ÷ 159 = 2, therefore the molecular formula is TWICE that of the EF – C20H14O4.