Empirical and Molecular Formula Determination
advertisement

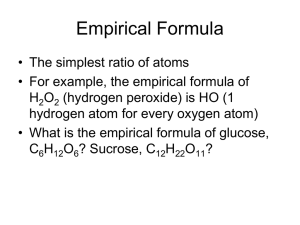
Chemical Formulas and the Mole Introduction • • • • • • • • • • • • 84 potatoes 63 carrots 15 onions 3 heads of garlic 27 turnips 42 pieces of celery 9 cans of green beans 6 cans of tomato puree 6 cans of diced peppers 9 cans of corn 6 cans of lima beans Serves 81 • If only 1 head of garlic is available, how many of each ingredient will be needed to adjust the recipe in order to make the stew correctly and how many people will it feed? • If you wanted to feed 324 people, how much of each ingredient would you need? How are formulas represented? • Calculate the % composition of the formula you were given. • % composition comes from experimental analysis and provides information about the make up of the compound. • Compare your calculations with the students around you having a different colored card. • What did you find regarding your % compositions? • Although the formulas are different, are there any similarities among your formulas that could account for the similarities of your calculations? Empirical Formulas • The molecular formula of a compound gives the actual number of atoms of each element making up a compound. • (The molecular formula is the formula that is written on your card) • The empirical formula of a compound is the formula with the smallest whole number ratio of the elements making up the compound. • What is the empirical formula for the compound written on your card? • The empirical formula may or may not be the same as the actual molecular formula. • If the two formulas are different, the molecular formula will always be a simple multiple of the empirical formula. Practice Problems • Write the empirical formula for each of the following molecular formulas. 1. C6H12O6 2. H2O2 3. C4H10 4. H2O 5. N2O4 6. C3H6O2 Determining Empirical Formulas • If % composition can be determined from the formula, then the formula can be determined from the % composition of the compound. Rules for determining Empirical Formulas 1. Assume a 100 g sample, so % becomes “g”. 2. Convert grams to moles by dividing by the atomic mass of each element. 3. Divide each result (mole) by the smallest result present (mole ratio). 4. Look for whole number ratios. 5. The whole number ratios become the subscripts for the formula. Rhyme for Remembering Rules “Percent to mass Mass to mole Divide by small Multiply 'til whole“ • A Simple Rhyme for a Simple Formula by Joel S. Thompson Journal of Chemical Education Vol. 65, No. 8; August 1988, p. 704 Sample Problems • Determine the empirical formula for a compound that is 27.3% carbon and 72.7% oxygen. • Determine the empirical formula for a compound that is 56.6% K, 8.7% C, and 34.7% O. • Determine the empirical formula for a compound that is 69.9% Fe and 30.1% O. Multiples for use when ratio is not initially a whole number Decimal Multiplier 0.5 X2 0.30-.35 X3 0.63 - .67 X3 0.22-0.25 X4 0.72-0.75 X4 Molecular Formulas • Molecular formulas are multiples of empirical formulas. Compound Empirical Formula Molar Mass Molecular Formula Formaldehyde CH2O 30 CH2O Acetic acid CH2O 60 C2H4O2 Glucose CH2O 180 C6H12O6 Sample Problems • The empirical formula for a compound containing phosphorus and oxygen was found to be P2O5. Experiments show that the molar mass of the compound is 284 g/mol. What is the molecular formula and name of the compound? • Determine the molecular formula of a compound having an empirical formula of CH and a molar mass of 78.11 g/mol. Putting it All Together • A compound with a molar mass of 92 g/mol contains 0.608 g of nitrogen and 1.388 g of oxygen. What is the empirical and molecular formula of the compound? • A compound with a molar mass of 86 g/mol contains 83.62% C and 16.38% H. What is the molecular formula of the compound? Hydrates • Hydrates are solid ionic compounds in which water molecules are trapped. • Some products, such as electronic equipment, are boxed with small packets labeled dessicant. These packets control moisture by absorbing water. Some contain ionic compounds called hydrates. • Each hydrate has a specific number of water molecules bound to its atoms. Example • An opal is hydrated silicon dioxide (SiO2) » » The presence of water and various mineral impurities accounts for the variety of colors. Naming Hydrates • In the formula for a hydrate, the number of water molecules associated with each formula unit of the compound is written following a dot. • For example: Na2CO3 ∙ 10 H2O • This compound is called sodium carbonate decahydrate. • The prefix deca- means ten and the root word hydrate refers to water. • When naming hydrates use the same prefixes for the water as used with covalent molecules. • A decahydrate has ten water molecules associated with each formula unit of compound. • The number of water molecules associated with hydrates varies widely. Examples of Hydrates Anhydrous (without water) CoCl2 The hydrate CoCl2 ∙ 6 H2O Hydrating CuSO4 Hydrated CuSO4 ∙ 5H2O Anhydrous CuSO4 Calculating the Formula Mass of Hydrates Na2CO3 ∙ 10 H2O Na: 2 x 23 = 46 C: 1 x 12 = 12 O: 3 x 16 = 48 H2O: 10 x 18 = 180 (H: 20 x 1 = 20; O: 10 x 16 = 160; 20 + 160 = 180) Total = 286 g/mol **Reminder-the “dot” in the formula means to add the mass of the water (not multiply as in math) Practice Problem Calculate the formula mass of the following: 1. CuSO4 ∙ 5 H2O Answer : 249.5 g/mol 2. BaCl2 ∙ 2 H2O Answer : 244 g/mol Determining the Formula of Hydrates from Experimental Data • The composition of a hydrate is determined to contain 48.8% MgSO4 and 51.2% H2O. • What is the formula of the hydrate? 48.8/120 (mass of MgSO4) = 0.407 moles MgSO4 51.2/18 (mass of H2O) = 2.84 moles H2O 0.407/0.407 = 1 2.84/0.407 = 7 Formula is MgSO4 ∙ 7 H2O Let’s Try Another • A 1.628 g sample of a sample of a hydrate of magnesium iodide (MgI2) heated until its mass is reduced to 1.072 g and all water has been removed. What is the formula of the hydrate? Answer: 1.072/278=0.00386 moles MgI2 1.628-1.072 = 0.556 g water/18 = 0.0309 moles H2O 0.00386/0.00386 = 1 0.0309/0.00386 = 8 Formula: MgI2 ∙ 8 H2O