Empirical Formulas
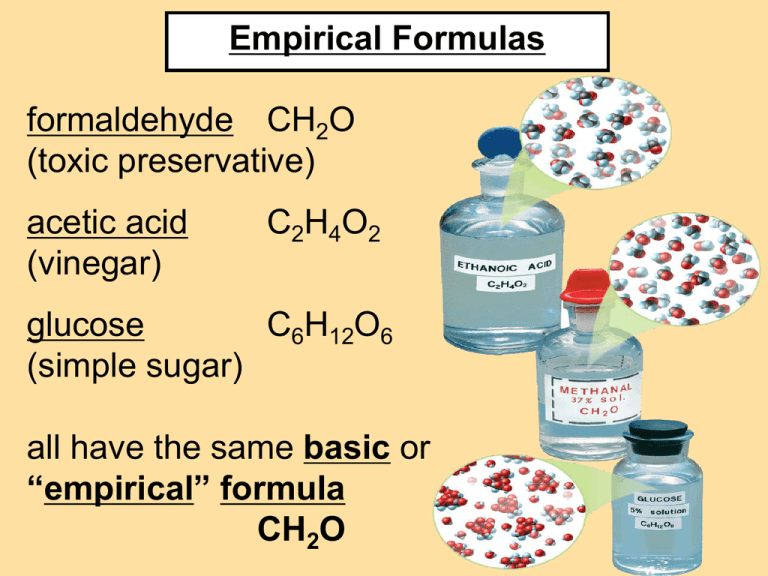
formaldehyde CH2O
(toxic preservative)
acetic acid
(vinegar)
C 2H 4O 2
glucose
C6H12O6
(simple sugar)
all have the same basic or
“empirical” formula
CH2O
© Copyright Pearson Prentice Hall
Slide
1 of 40
End Show
Percent Composition and
Chemical Formulas
>
empirical formula:
the smallest whole-number ratio of
the atoms in a compound.
Examples:
CH3
HO
PbO2
C2H6N
Pb2O4
C6H18N3
Non-Examples:
C 2H 6
H 2O 2
© Copyright Pearson Prentice Hall
Slide
2 of 40
End Show
Percent Composition and
Chemical Formulas
>
Acetylene (C2H2) is a gas
used in welder’s torches.
These two compounds of
carbon both have the same
empirical formula (CH) but
different molecular formulas.
Styrene (C8H8) is used in
making polystyrene.
© Copyright Pearson Prentice Hall
Slide
3 of 40
End Show
SAMPLE
PROBLEM
What
is the
empirical formula of a
compound that is analyzed and found to
contain 25.9% N and 74.1% O?
Percent to Mass
Mass to Mole
Divide by Small
Times ‘till Whole
© Copyright Pearson Prentice Hall
Slide
4 of 40
End Show
SAMPLE
PROBLEM
What
is the
empirical formula of a
compound that is analyzed and found to
contain 25.9% N and 74.1% O?
Percent to Mass
Mass to Mole
Divide by Small
Times ‘till Whole
25.9 g N x 1 mol N = 1.85
mol N = 1 N
________
14.01 g N
1.85
x2=2N
74.1 g O x 1 mol O = 4.63
mol O = 2.5 O x 2 = 5 O
________
16.00 g O
Slide
1.85
5 of 40
© Copyright Pearson Prentice Hall
N2OEnd
5 Show
1,6-diaminohexane is used to make
nylon. What is the empirical formula if it
is 62.1% C, 13.8% H, and 24.1% N?
Practice Problems
62.1 g C x 1 mol C = 5.17
mol C
________
12.01 g C
1.72
= 3C
13.8 g H x 1 mol H = 13.66
mol H = 8 H
_________
1.01 g H
1.72
24.1 g N x 1 mol N = 1.72
mol N
________
14.01 g N
1.72
= 1N
C 3H 8N
© Copyright Pearson Prentice Hall
Slide
6 of 40
End Show
Percent Composition and
Chemical Formulas
>
molecular formula:
a whole-number multiple of the
empirical formula of a compound
formaldehyde CH2O
acetic acid
C 2H 4O 2
glucose
C6H12O6
all have the same
empirical formula
CH2O
© Copyright Pearson Prentice Hall
Slide
7 of 40
End Show
SAMPLE PROBLEM
Calculate
the molecular formula of a
compound with the empirical formula
CH4N and a molar mass of 60.0 g/mol.
molecular mass = multiple of emp. form.
empirical mass
empirical mass
1(C) + 4(H) + 1(N) =
12.01 + 4(1.01) + 14.01 = 30.06 g/mol
60.0 = 1.996 ≈ 2
30.06
2 (CH4N) =
© Copyright Pearson Prentice Hall
C 2H 8N 2
Slide
8 of 40
End Show
SAMPLE PROBLEM
Calculate
the molecular formula of
benzene with the empirical formula CH
and a molar mass of 78 g/mol.
molecular mass = multiple of emp. form.
empirical mass
empirical mass
1(C) + 1(H) =
12.01 + 1.01 = 13.02 g/mol
78 = 5.991 ≈ 6
13.02
6 (CH) =
© Copyright Pearson Prentice Hall
C 6H 6
Slide
9 of 40
End Show
Quick Quiz!
1. An empirical formula shows the __________
of atoms in a compound.
A. lowest common number
B. highest whole ratio
C. lowest whole number ratio
D. average ratio
Quick Quiz.
2. Which of the following is NOT an empirical
formula?
A. CH3
B. H2N
C. CH
D. C3H6
Quick Quiz.
3. True or False:
A molecular formula can be the same as an
empirical formula.
True
False
Example:
Formaldehyde has the
empirical formula CH2O,
which is its molecular formula as well.
Quick Quiz.
4. Determine the molecular formula of a
compound that contains 40.0 % C, 6.71 % H,
and 53.29 % O by mass and has a molar
mass of 60.05 g/mol.
A. C2H4O2
B. CH2O
C. C2H3O
D. C2H4O