Chapter 10 - Rose
advertisement

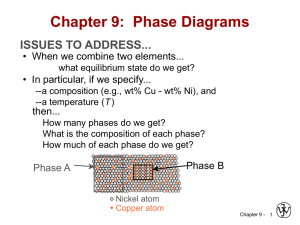
Materials Engineering – Day 13 More About Steel Chapter 10 - Steel and Carbon Conent • 1015 steel – plain carbon – 0.15%C • 1090 steel – plain carbon – 0.90%C • What happens as carbon content increases? In general, we see more and more pearlite in slow cooled steels. More and more cementite available in all steels. Strength up. Ductility down. • BUT, AT A GIVEN CARBON CONTENT, WIDELY VARYING PROPERTIES ARE AVAILABLE DEPENDING ON PROCESSING. Chapter 10 - Chapter 10: Phase Transformations ISSUES TO ADDRESS... • Transforming one phase into another takes time. Fe g (Austenite) C FCC Fe C 3 Eutectoid transformation (cementite) + a (ferrite) (BCC) • How does the rate of transformation depend on time and T? • How can we slow down the transformation so that we can engineering non-equilibrium structures? • Are the mechanical properties of non-equilibrium structures better? Chapter 10 - 3 The Austenite to Pearlite Transformation • This takes time. Two things must happen. 1. Nucleation of Pearlite. (Best nucleation sites are in or near grain boundaries.) Nuclei are baby crystals. The thermodynamics of nucleation get stronger with supercooling. 2. Growth of Pearlite. This takes diffusion. Carbon atoms must diffuse away from the g to the Fe3C layers which are starting. Diffusion is stronger at high temperatures and weakens as the temperature decreases. • These two aspects of the transformation work against each other. Chapter 10 - Eutectoid Transformation Rate • Growth of pearlite from austenite: Adapted from Fig. 9.15, Callister 7e. a a g a a a a • Recrystallization rate increases with DT. g cementite (Fe3C) Ferrite (a) a g a pearlite growth direction g a 100 y (% pearlite) Austenite (g) grain boundary Diffusive flow of C needed 600°C (DT larger) 50 650°C 675°C (DT smaller) Adapted from Fig. 10.12, Callister 7e. 0 Course pearlite formed at higher T - softer Fine pearlite formed at low T - harder Chapter 10 - 5 C-Curves • Think of some austenite, lowered suddenly to a temperature below 727C and allowed to transform at that temperature. • At high temperature, atoms can diffuse rapidly BUT, nucleation rates are very low due to only slight undercooling. Therefore, the overall transformation tends to be lengthy. • At low temperature, nucleation is very speedy, but diffusion is slow. Therefore the transformation tends to be lengthy. • At some intermediate temperatures there must be an optimum. Thus we get a C-shaped curve. Chapter 10 - Nucleation and Growth • Reaction rate is a result of nucleation and growth of crystals. 100 % Pearlite Nucleation rate increases with DT Growth regime 50 Nucleation Growth rate increases with T regime t 0.5 0 log (time) Adapted from Fig. 10.10, Callister 7e. • Examples: g pearlite colony T just below TE Nucleation rate low Growth rate high g T moderately below TE Nucleation rate med . Growth rate med. g T way below TE Nucleation rate high Growth rate low Chapter 10 - 7 TTT Curves for a Euctectoid Steel Note the axes: 1. Time – always log scale. 2. Temperature Note the curves: 1. Start time 2. 50% Completion (dashed) 3. End time. (after crossing this there is no more austenite left to transfform. Chapter 10 - Isothermal Transformation Diagrams y, % transformed • Fe-C system, Co = 0.76 wt% C Note the axes: • Transformation at T = 675°C. 1. Time – always log 100 scale. T = 675°C 50 2. Temperature 0 Note the curves: 1. Start time T(°C) Austenite (stable) 2. 50% Completion T (727C) E 700 Austenite (dashed) (unstable) Pearlite 3. End time. (after 600 isothermal transformation at 675°C crossing this there is 500 no more austenite 400 left to transform. 10 2 1 1 10 10 4 time (s) 10 2 10 3 10 4 10 5 time (s) Chapter 10 - 9 The Pearlite Spectrum • The kind of pearlite that you get depends on the transformation temperature. 1. High temperature. Low nucleation. Higher growth. Larger coarser more separated layers in the pearlite. Not as strong, more ductile. 2. Low temperature. (Nearer to the “nose.”) High nucleation. Little diffusion. Finer thinner closer spaced layers in the pearlite. Stronger, less ductile. 3. Intermediate temperatures – an intermediate pearlite. Chapter 10 - Two Pearlites. Which formed at the higher Temperature? Which is stronger? Which is more ductile? Chapter 10 - What Happens as we Transform at Lower and Lower Temperatures? T (C) 727 Fine P ??? Coarse P Log time Chapter 10 - The Answer is “Bainite” • Bainite is relatively rare. The austenite is changed to pearlite before the bainite transformation can start in most practical cooling processes. But it is good stuff. Strong, hard, some ductility. Upper (above 300) Lower (below 300) Upper B. Needles of ferrite with strips of cementite Lower B. Very thin ferrite plates. Ultra thin cementite rods Chapter 10 - What Happens if we Transform at an even lower Temperature? • The answer is that we get a phase known as martensite. • Martensite is BC tetragonal with C interstitially. • It forms instantaneously. No nucleation or diffusion is required. • Martensite is one of the hardest substances known to man. It is also totally brittle like a ceramic. • The production of martensite is an important step, but not the final step, in the production of high strength steels. (Quench and tempered) Chapter 10 - Here is the TTT Curve for a Euctectoid Steel. Coarse P Fine P Upper B Lower B M (Martensite) Chapter 10 - Martensite: Fe-C System • Martensite: --g(FCC) to Martensite (BCT) Fe atom sites x x x x x 60 m (involves single atom jumps) potential C atom sites x (Adapted from Fig. 10.20, Callister, 7e. • Isothermal Transf. Diagram 800 Austenite (stable) T(°C) A 400 10-1 (Adapted from Fig. 10.21, Callister, 7e. (Fig. 10.21 courtesy United States Steel Corporation.) • g to M transformation.. B A 200 TE P 600 Adapted from Fig. 10.22, Callister 7e. Martensite needles Austenite 0% 50% 90% M+A M+A M+A 10 103 105 -- is rapid! -- % transf. depends on T only. time (s) Chapter 10 - 16 Martensite Formation g (FCC) slow cooling a (BCC) + Fe3C quench M (BCT) tempering M = martensite is body centered tetragonal (BCT) Diffusionless transformation BCT few slip planes BCT if C > 0.15 wt% hard, brittle Chapter 10 - 17 Tempering Martensite • reduces brittleness of martensite, • reduces internal stress caused by quenching. TS(MPa) YS(MPa) 1800 Adapted from 1400 Fig. 10.34, Callister 7e. (Fig. 10.34 1200 adapted from Fig. furnished 1000 courtesy of Republic Steel Corporation.) 800 200 TS YS 60 50 %RA 40 30 %RA 400 9 m 1600 Adapted from Fig. 10.33, Callister 7e. (Fig. 10.33 copyright by United States Steel Corporation, 1971.) 600 Tempering T (°C) • produces extremely small Fe3C particles surrounded by a. • decreases TS, YS but increases %RA Chapter 10 - 18 Mechanical Prop: Fe-C System (1) • Effect of wt% C Adapted from Fig. 9.30,Callister 7e. (Fig. 9.30 courtesy Republic Steel Corporation.) TS(MPa) 1100 YS(MPa) Co < 0.76 wt% C Hypoeutectoid Hypo Hyper Co > 0.76 wt% C Adapted from Fig. 9.33,Callister 7e. 9.33 copyright 1971 by United Hypereutectoid (Fig. States Steel Corporation.) %EL Hypo Hyper 80 100 900 hardness 40 700 50 500 0 0.5 1 0 Adapted from Fig. 10.29, Callister 7e. (Fig. 10.29 based on data from Metals Handbook: Heat Treating, Vol. 4, 9th ed., V. Masseria (Managing Ed.), American Society for Metals, 1981, p. 9.) 0.76 0 0.76 300 Impact energy (Izod, ft-lb) Pearlite (med) ferrite (soft) Pearlite (med) Cementite (hard) 1 0.5 0 wt% C wt% C • More wt% C: TS and YS increase, %EL decreases. Chapter 10 - 19 Mechanical Prop: Fe-C System (2) • Fine vs coarse pearlite vs spheroidite Hypo Hyper 90 Hypo Hyper fine pearlite 240 coarse pearlite spheroidite 160 80 0 • Hardness: • %RA: 0.5 1 wt%C Ductility (%AR) Brinell hardness 320 spheroidite 60 coarse pearlite fine pearlite 30 0 0 fine > coarse > spheroidite fine < coarse < spheroidite 0.5 1 wt%C Adapted from Fig. 10.30, Callister 7e. (Fig. 10.30 based on data from Metals Handbook: Heat Treating, Vol. 4, 9th ed., V. Masseria (Managing Ed.), American Society for Metals, 1981, pp. 9 and 17.) Chapter 10 - 20 Mechanical Prop: Fe-C System (3) • Fine Pearlite vs Martensite: Brinell hardness Hypo 600 Hyper martensite Adapted from Fig. 10.32, Callister 7e. (Fig. 10.32 adapted from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 36; and R.A. Grange, C.R. Hribal, and L.F. Porter, Metall. Trans. A, Vol. 8A, p. 1776.) 400 200 fine pearlite 0 0 0.5 1 wt% C • Hardness: fine pearlite << martensite. Chapter 10 - 21 Summary: Processing Options Austenite (g) slow cool moderate cool Adapted from Fig. 10.36, Callister 7e. rapid quench Bainite Martensite (a + Fe3C layers + a proeutectoid phase) (a + Fe3C plates/needles) (BCT phase diffusionless transformation) Martensite T Martensite bainite fine pearlite coarse pearlite spheroidite General Trends reheat Ductility Strength Pearlite Tempered Martensite (a + very fine Fe3C particles) Chapter 10 - 22 Spheroidite: Fe-C System • Spheroidite: a (ferrite) --a grains with spherical Fe3C --diffusion dependent. --heat bainite or pearlite for long times Fe3C --reduces interfacial area (driving force) (cementite) 60 m (Adapted from Fig. 10.19, Callister, 7e. (Fig. 10.19 copyright United States Steel Corporation, 1971.) Chapter 10 - 26 Phase Transformations of Alloys Effect of adding other elements Change transition temp. Cr, Ni, Mo, Si, Mn retard g a + Fe3C transformation Adapted from Fig. 10.23, Callister 7e. Chapter 10 - 27 Cooling Curve plot temp vs. time Adapted from Fig. 10.25, Callister 7e. Chapter 10 - 28 Dynamic Phase Transformations On the isothermal transformation diagram for 0.45 wt% C Fe-C alloy, sketch and label the time-temperature paths to produce the following microstructures: a) 42% proeutectoid ferrite and 58% coarse pearlite b) 50% fine pearlite and 50% bainite c) 100% martensite d) 50% martensite and 50% austenite Chapter 10 - 29 Example Problem for Co = 0.45 wt% a) 42% proeutectoid ferrite and 58% coarse pearlite first make ferrite 800 then pearlite T (°C) A P B 600 course pearlite higher T A+a A+P A+B A 400 50% M (start) M (50%) M (90%) 200 Adapted from Fig. 10.29, Callister 5e. 0 0.1 10 103 time (s) 105 Chapter 10 - 30 Example Problem for Co = 0.45 wt% b) 50% fine pearlite and 50% bainite 800 first make pearlite T (°C) then bainite A P B 600 fine pearlite lower T A+a A+P A+B A 400 50% M (start) M (50%) M (90%) 200 Adapted from Fig. 10.29, Callister 5e. 0 0.1 10 103 time (s) 105 Chapter 10 - 31 Example Problem for Co = 0.45 wt% c) 100 % martensite – quench = rapid cool d) 50 % martensite 800 A+a and 50 % A T (°C) austenite P B 600 A+P A+B A 400 50% M (start) M (50%) M (90%) d) 200 Adapted from Fig. 10.29, Callister 5e. c) 0 0.1 10 103 time (s) 105 Chapter 10 - 32