Pituitary Incidentalomas
ENDOCRINOLOGY ROUNDS
KRISTIN CLEMENS PGY 5
N O V E M B E R 7 TH, 2 0 1 2
Objectives
Epidemiology of pituitary incidentalomas
Underlying causes
Outline understanding of natural history
Review recent practice guidelines on pituitary
incidentalomas
Illustrative cases for work up and management
Case – E.A.
70 year old man
HTN, hypercholesterolemia
Diovan, Crestor
No family history
Non smoker, rare ETOH, lives independently with
wife
Acute onset memory impairment – ER
Diagnosis of transient global amnesia
CT to rule out stroke demonstrated 2 x 1.9 x 1.6 cm
pituitary macroadenoma
MRI encroachment of left cavernous sinus, touching
L optic nerve
Pituitary MRI
How to assess him at follow-up endocrinology
appointment?
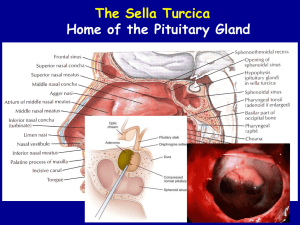
Pituitary incidentaloma
Previously unsuspected pituitary lesion discovered
on an imaging study performed for an unrelated
reason
Increasingly common
Prevalence
Estimated from pituitary adenomas found at autopsy
and from imaging inpatients who underwent CT or
MRI
Combined autopsy data suggests frequency of 10.6%,
distributed equally amongst genders, age range
MRI and CT incidence of 4-38%
Etiology
Rathke cleft cysts
Craniopharyngiomas
Meningiomas
Pituitary hyperplasia
Metastases
Pituitary adenoma – 10%
Macroadenoma >1cm
Microadenoma <1 cm, most common
Functioning and non-functioning
Pituitary Adenomas
Variety of subtypes
Data from small observational studies
Buurman, 2006
Series of 3048 autopsy cases examined to detect incidence of
subclinical adenomas in postmortem pituitaries
1991-2004
Buurman et al
Found a total of 334 adenomas out of 3048 cases
(10.4%) examined
157 males and 159 females
Mean age of 73
Size range from 0.1 to 20 mm
Only 3 were macroadenomas
Some with multiple adenomas
Multiple staining techniques
39.5% stained positive for prolactin
31.7% null cell adenoma/oncocytomas
13.8% stained positive for ACTH
7.2% for gonadotropins
1.8% stained positive for GH
0.6% for TSH
3% for multiple hormones
Small percentage unclassified
Clinical history
Clinical significance?
99 patients with HTN, 65 with diabetes
No symptoms of adenohypophyseal hormone
hypersecretion were reported
Rare cases of clinical hypo and hyperthyroidism
No clear correlation between clinical data and
adenoma type in this sample
Natural History?
Small observational cohort studies
Fernandez-Balsells, 2011
Systematic review of longitudinal observational
cohort studies that enrolled patients with:
Incidentalomas (asymptomatic lesions)
Non-functioning pituitary adenomas (mass
effect/hypopituitarism)
Followed without any treatment from the time of
detection
Outcomes of interest including development of
visual field defects, neurological defects, alteration in
pituitary function, pituitary apoplexy
MEDLINE, EMBASE, Cochrane from 1966 through
2009
Abstraction of data in duplicate
Event rate per 100 person years and associated 95%
confidence interval
Subgroup analysis specified a priori according to
tumour size at presentation (micro vs.
macroadenoma), tumour characteristics (solid vs.
cystic lesion), patients age and sex
Results
14 publications from 1069 references
Small studies
Majority single centre studies
Limited quality
Often >30 % lost to follow up
Median follow up of 3.9 years (range 1-15 years)
Frequency of PI/NFPA’s higher in females
Wide age range 5 months to 89 years
Among symptomatic patients most common
complaint was headache at baseline
Most common pituitary dysfunction at baseline was
hypogonadism
Outcomes
Outcomes reported in aggregate as unable to
differentiate between incidentalomas and nonfunctioning pituitary macroadenomas
Event rate per 100 PY’s
Tumour growth 5.8 (5-6.5)
Pituitary apoplexy 0.2 (0.0-0.2)
Worsening of visual field defects 0.7 (0.5-0.8)
Overall incidence of new endocrine dysfunction 2.4 (0-6.4)
Significant heterogeneity
A priori subgroup analysis
Greater event rate of growth in size in
macroadenomas (12.5) compared with
microadenomas (3.3) and cystic lesions (0.1)
Pituitary Incidentaloma: An Endocrine Society
Clinical Practice Guideline
2011
Consensus guided by systematic review of evidence
and expert opinion
Evidence developed using Grading of
Recommendations, Assessment, Development and
Evaluation (GRADE) system to describe strength of
recommendations and quality of evidence
+Very low quality
++++ High quality
Initial evaluation of a patient with a pituitary
incidentaloma?
Recommendation 1.1.1 (+++)
All patients with a pituitary incidentaloma should
undergo complete history and physical and
evaluation for hormone hypersecretion
What hormones to be assessed?
Prolactin
Adenoma, stalk compression
Recommended as hyperprolactinemia treatable
medically
Growth hormone
Generally recommended as silent growth hormone
secretion has been reported
Medical comorbidity
ACTH
Variable recommendations
Cushing's with significant morbidity and risk of
progression to overt Cushing’s
One small study noted progression to overt Cushing
disease in 4/22 or 18% of cases
Focused assessment of gonadotropins
High gonadotropins rarely cause clinical symptoms so
screening would not necessarily influence therapy
Focused assessment of TSH
Rare for TSH secreting adenomas
If hormonal overproduction treat medically or
surgically as per guidelines
Recommendation 1.1.2 (+++)
We recommend that patients with a pituitary
incidentaloma with or without symptoms also
undergo clinical and laboratory evaluations for
hypopituitarism
Systematic review
Hypopituitarism in 7/66 (10%) and 19/46 (40%)
patients in smaller observational studies
Hypogonadism in 30%
Hypothyroidism 28%
Adrenal insufficiency in 18%
Growth hormone insufficiency in 8%
Favoured testing in macroadenomas but variable
amongst contributors
Recommendation 1.1.3 (++++)
We recommend that all patients presenting with a
pituitary incidentaloma abutting or compressing the
optic nerves or chiasm on MRI undergo formal visual
field testing
Growth may lead to sight loss
5-15% may have unrecognized visual fields at
presentation
Recommendation 1.1.4 (++++)
We recommend that all patients have a MRI scan to
better delineate the nature and extent of the
incidentaloma
Specific pituitary protocol with fine cuts through the
sella
Follow-up testing of the pituitary incidentaloma?
Recommendation 2.1 (++)
Patients with incidentalomas who do not meet
criteria for surgical removal, should receive non
surgical follow-up with:
2.1.1 MRI scan 6 months after initial scan for
macroadenoma or 1 year if microadenoma (++)
Repeat MRI every year for macroadenoma
MRI every 1-2 years for microadenoma for 3 years and then
less frequently
Systematic review on natural history
2.1.2 (++++)
Visual field testing in those with an incidentaloma
that enlarges to abut the chiasm or optic nerves
2.1.3 (++)
Clinical and biochemical evaluation for
hypopituitarism 6 months after the initial testing
and 1 year thereafter in macroadenoma
Follow up to continue for several years
Indications for surgical therapy of the pituitary
incidentaloma?
Recommendation 3.1 (++++)
Refer for surgery if:
Evidence of VF defect or other visual field
abnormalities
Relatively higher value put on prevention of VF abnormalities
than on avoiding the morbidity (hypopituitarism) and cost of
the surgery
Lesion abutting or compressing the optic nerves or
chiasm on MRI
Pituitary apoplexy
Hypersecreting tumours other than prolactinomas
Recommendation 3.2 (++)
Surgery if:
Clinically significant growth of the incidentaloma
Loss of endocrine function
A lesion close to the chiasm with a plan to become
pregnant
Unremitting headache
Transphenoidal approach favoured
Algorithm
Although guidelines, variable practice among
clinicians
Given prevalence of pituitary adenomas, cost
concern
Randall et al, 2010
Single institution review of patients with pituitary
tumour, adenoma, Cushing disease, acromegaly,
prolactinoma
2002-2009
Pituitary tumours that were discovered incidentally
were about 15% of sample
King et al, 1997
Cost effective analysis using Markov modeling to
compare strategies for management of asymptomatic
patient with incidental pituitary microadenoma
Expectant- no management unless symptoms
PRL screening with treatment
Panel of hormone secreting tests – PRL, IGF 1,
cortisol after dexamethasone administration with
treatment
Follow up MRI screening – 6 and 12 months with
hormone testing if size change
Incorporated natural history, pharmacological and
surgical treatment outcomes
Direct medical costs - costs of hormone testing,
MRI’s, hospitalization for surgery and physician
services into analysis
Mortality, morbidity from endocrine and
neurological dysfunction, anxiety about knowing
about the tumour
Quality of life (QALY)- measure of quality of life that
assigns to each year, a weight of quality of life
Results most sensitive to patient anxiety and shifts to
endocrine panel
Concluded that single PRL may be most cost
effective screen for microadenoma
Thus….
Some groups suggest only prolactin for
hyperfunction and other hormonal work up
depending on clinical suspicion
No routine visual field testing
No routine screening for hormone hypofunction
Back to E.A
History
No headaches, visual impairment, facial weakness or
parasthesias
No overt symptoms of hormone hyperfunction
No symptoms of hypofunction
Physical exam
No orthostatic change in vitals
Normal visual fields to confrontation, normal
extraocular movements
Clinical euthyroid, no features of acromegaly,
Cushing’s, well-androgenized
Clinical suspicion for hormone dysfunction low
Investigations
Cortisol 498 nmol/L
LH 4.8 IU/L, FSH 12.9 IU/L, total testosterone 14
nmol/L
Prolactin 5 ug/L
TSH 1.57 mIU/L, free T4 15 pmol/L, free T3 4.4
pmol/L
IGF 1 normal
Visual fields unreliable with multiple false positives
and negatives
Repeat examination normal
Follow-up
Repeat MRI at 6 months stable
Repeat MRI at 1 year stable
Plan to repeat visual fields and assess for hormone
hypofunction
Case 2 – Z.K.
78 year old man
CVA, HTN
“Blood pressure pill”
No family history
Non smoker, no alcohol, lives independently with
wife
Presented to ER with history of headache
CT showed 3.1 x 2.8 x 3.8 cm macroadenoma
MRI demonstrated suprasellar extension and
extension into the sphenoid sinus and R cavernous
sinus
Mild compression of the optic chiasm
Pituitary MRI
Endocrine follow-up
Son translator
Headaches improved
Denied vision impairment, facial weakness or
parasthesia
No symptoms of pituitary hyperfunction
?Cold intolerance – multiple layers of clothing
around the house
Physical exam
No orthostatic change in vitals, no lightheadedness
Visual fields difficult to assess
No features of Cushing’s or acromegaly, well
androgenized
Wearing double layers and hat in summer
Investigations
AM cortisol 144 nmol/L
LH 1 IU/L, FSH 3.1 IU/L, total testosterone <0.1
nmol/L
TSH 1.14 mIU/L, free T4 9 pmol/L, free T3 3.5
pmol/L
Prolactin 21 ug/L
IGF 1 <15 ug/L
What next?
Started hydrocortisone 20 mg q am and 10 mg q pm
Levothyroxine 75 micrograms po daily
BMD and consideration of testosterone replacement
Growth hormone replacement?
Case
Visual field examination unreliable as language
barrier
Suggested ongoing MRI’s for follow up
Repeat MRI at 6 months stable
Plan for 1 year repeat exam
Low threshold for surgical management
Case #3 – R. J.
50 year old lady
Colonic polyps, hypercholesterolemia, thyroid
nodule
Previous hysterectomy
Family history of DMII
Previous smoker
ENT for feelings of oropharyngeal swelling and
shortness of breath
Referred for “incidental” 14 x 21 x 21 mm
macroadenoma on sinus CT
MRI mild stalk deviation, suprasellar extension
Encroachment of right optic nerve without
compression
MRI
History
Increased shoe size and hand size over last several
year
20 lb weight gain
Flushing
Snoring
Voice deepening
No galactorrhea
No symptoms suggestive of hormone insufficiency
Physical Exam
No orthostatic change in blood pressure
Normal visual fields to confrontation
Several clinical manifestations of acromegaly
Investigations
ACTH, cortisol normal
LH 2.2 IU/L, FSH 4.7 IU/L, estradiol 164 pmol/L
TSH 0.93 mIU/L, fT4 14 pmol/L, fT3 5.9 pmol/L
Prolactin 40 ug/L
GH 35 ug/L, IGF 1 711 ug/L
Management
75 g OGTT confirmed acromegaly
Visual field testing normal
Referral for surgical management
Take home messages
With increased imaging, more pituitary
incidentalomas
Relatively common endocrinology referral
Pituitary adenomas among other etiologies
Clinical guidelines helpful but need to consider
individual patient
Detailed history and physical
Screen for hormone hyperfunction – prolactin, IGF
1, others if clinically suspicious
Screen for hormone hypofunction – macroadenomas
Refer for visual field assessment if close to the optic
chiasm
Consider medical or surgical management
Long term monitoring for clinical changes
References
Buurman H, Saeger W. Subclinical adenomas in
postmortem pituitaries: classification and
correlations to clinical data. European Journal of
Endocrinology 2006; 154: 753-758.
King JT et al. Management of incidental pituitary
microadenomas: a cost effectiveness analysis. JCEM
1997; 82: 3625-3632.
Pituitary Incidentaloma: An Endocrine Society
Clinical Practice Guideline 2011
Randall BR et al. Cost of evaluation of patients with
pituitary incidentaloma. Pituitary 2010; 13: 383-384.
Thanks!