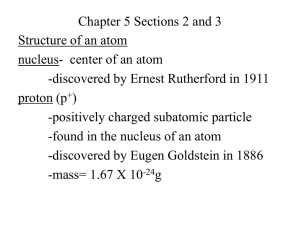
the structure of an atom
advertisement

Atomic Structure: TEKS 8.5A To the teacher: • This CPO Science PowerPoint presentation is designed to guide you through the process of presenting the lesson to your students. The presentation uses a 5-E teaching model: Engage, Explore, Explain, Elaborate, and Evaluate. • The PowerPoint Slide notes indicate where you may want to bring in various lesson elements such as quizzes, readings, investigations, animations, and practice materials. Additional science background information is provided in the slide notes where appropriate. You can view these notes by selecting “View,” then “Normal.” You will see the notes pane at the bottom of the PowerPoint workspace. Additionally, the slide notes are available as a separate document, accessible from the lesson home page. • The slides that follow are intended for classroom use. Atomic Structure: TEKS 8.5A What is inside an atom? • If you crush wintergreen candy with your teeth, blue sparks jump out of your mouth! You can see the effect even better if you crush one of the candies with a pair of pliers in a dark room. • In order to understand why the sparks appear, you must know what is inside an atom. After completing this module, do an internet search on triboluminescence to find out why the candy sparks when you crush it. Atomic Structure: TEKS 8.5A Time to investigate! • Complete the lesson investigation: Building the Elements Atomic Structure: TEKS 8.5A Three particles make up all atoms. • Protons have positive (+1) charge. They are found in the nucleus of every atom. • Neutrons have zero charge. They are also found in the nucleus of every atom except hydrogen. • Electrons have negative (-1) charge. They are found in an area outside the nucleus known as the electron cloud. Atomic Structure: TEKS 8.5A The size and structure of an atom • The overall size of an atom is the size of its electron cloud. • If an atom were the size of a football stadium, the nucleus would be the size of a pea, and the electron cloud would be like a small swarm of gnats buzzing around the stadium at high speed. Atomic Structure: TEKS 8.5A Where is most of an atom’s mass? • A proton has 1,836 times as much mass as an electron. • A neutron has about the same mass as a proton. • More than 99% of an atom’s mass is in the nucleus. Atomic Structure: TEKS 8.5A Summary: the structure of an atom Atomic Structure: TEKS 8.5A Time for Practice! • Complete the lesson practice activity: – Structure of the atom You will need to know what these words mean: atomic number mass number isotope Review your student reading for help! Atomic Structure: TEKS 8.5A Show what you know! • Try the lesson’s interactive quiz, or complete a quiz that your teacher can print out for you. • Hint: You might want to review your lesson reading piece one more time before trying the quiz.