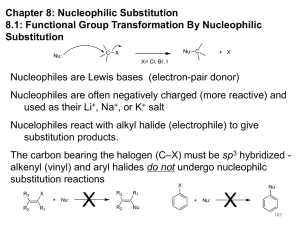
Ch10-Sn1 - ChemConnections

Unimolecular Nucleophilic Substitution
S
N
1
A question...
Tertiary alkyl halides are very unreactive in substitutions that proceed by the S
N
2 mechanism.
Do they undergo nucleophilic substitution at all?
Yes. But by a mechanism different from S
N
2.
The most common examples are seen in solvolysis reactions.
The Sn1 Reaction
CH
3
CH
3
C
Example of a solvolysis. Hydrolysis of tert -butyl bromide.
..
: + : O :
H
CH
3
H
CH
3
CH
3
..
C OH
CH
3
+
..
H Br
..
:
Kinetics and Mechanism rate = k [alkyl halide]
First-order kinetics implies a unimolecular rate-determining step.
Mechanism is called S
N
1, which stands for substitution nucleophilic unimolecular (1)
CH
3
CH
3
C
CH
3
..
:
H
3
C
+
C unimolecular slow
CH
+
3
:
..
Br :
–
CH
3
Mechanism
H
3
C
+
C
CH
3
CH
3
: O :
H
H
bimolecular fast
CH
3
CH
3
C
+
O :
H
CH
3
H
Mechanism
carbocation formation
R + carbocation capture proton transfer
RX
+
ROH
2
ROH
Characteristics of the S
N
1 mechanism
• First order kinetics: rate = k [RX] unimolecular rate-determining step
• Carbocation intermediate rate follows carbocation stability rearrangements are observed
• Reaction is not stereospecific: racemization in reactions of optically active alkyl halides
Reaction Coordinate Diagram for an
S
N
1 Reaction
Carbocation Stability and S
N
Rates
1 Reaction
Electronic Effects Govern S
N
1 Rates
The rate of nucleophilic substitution by the S
N
1 mechanism is governed by electronic effects.
Carbocation formation is rate-determining.
The more stable the carbocation, the faster its rate of formation, and the greater the rate of unimolecular nucleophilic substitution.
Reactivity toward substitution by the S
N
1 mechanism
RBr solvolysis in aqueous formic acid
Alkyl bromide Class
CH
3
Br
CH
3
CH
2
Br
(CH
3
)
2
CHBr
(CH
3
)
3
CBr
Methyl
Primary
Secondary
Tertiary
Relative rate
1
2
43
100,000,000
Decreasing S
N
1 Reactivity
(CH
3
)
3
CBr
(CH
3
)
2
CHBr
CH
3
CH
2
Br
CH
3
Br
Stereochemistry of S
N
1 Reactions
Generalization
Nucleophilic substitutions that exhibit first-order kinetic behavior are not stereospecific.
Stereochemistry of an S
N
1 Reaction
CH
3
H
R -( –)-2-Bromooctane C Br
CH
3
(CH
2
)
5
HO C
H
CH
3
(CH
2
)
5
CH
3
( S )-(+)-2-Octanol (83%)
H
2
O
CH
CH
3
3
(CH
H
2
C
)
5
OH
( R )-( –)-2-Octanol (17%)
The carbocation reaction intermediate leads to the formation of two stereoisomeric products
Step 1
+
Ionization step gives carbocation; three bonds to stereogenic center become coplanar
Step 2
+
Leaving group shields one face of carbocation; nucleophile attacks faster at opposite face.
More than 50%
+
Less than 50%
Carbocation Rearrangements in S
N
1 Reactions
Because...
carbocations are intermediates in S
N
1 reactions, rearrangements are possible.
Carbocations
Carbocations rearrange to the more stable form(s)
Carbocation Rearrangement
Mechanism
What is the starting carbocation: 1 o , 2 o or 3 o ?
What is the rearranged carbocation: 1 o , 2 o or 3 o ?
Carbocation Rearrangement
CH
3
CH
3
C CHCH
3
H Br
Example
H
2
O
CH
3
CH
3
C
OH
CH
2
CH
3
(93%)
CH
3
CH
3
C CHCH
3
H Br
CH
3
CH
3
C CHCH
3
+
H
Example
CH
3
CH
3
CH
3
C
OH
CH
2
CH
3
(93%)
H
2
O
CH
3
C CHCH
3
+
H
Mechanism Summary
S
N
1 and S
N
2
When...
primary alkyl halides undergo nucleophilic substitution, they always react by the S
N
2 mechanism tertiary alkyl halides undergo nucleophilic substitution, they always react by the S
N
1 mechanism secondary alkyl halides undergo nucleophilic substitution, they react by the
S
N
1 mechanism in the presence of a weak nucleophile (solvolysis)
S
N
2 mechanism in the presence of a good nucleophile