SN-2, SN-1, E-2 dan E-1
advertisement

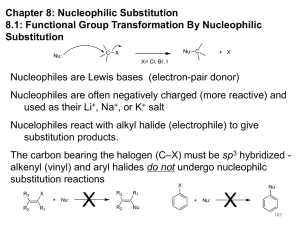
Substitution and Elimination Reaction of Alkyl Halides By: Ismiyarto, MSi 1 ALKIL HALIDA 1. Manfaat (Pestisida, Bahan Sintesis Alkohol, Alkena) 2. Struktur (Metil, Primer, Tersier, Benzil dan Vinil) Dasar Sekunder, 3. Reaksi (SN-2, SN-1, E-2 dan E-1) 2 PETA REAKSI ALKIL HALIDA 1. 2. 3. 4. Metil Halida Alkil halida Primer Alkil Halida Sekunder Alkil Halida Tersier 5. 6. Alil Halida Benzil Halida SN-2 SN-2 SN-2, SN-1 dan E-2 SN-2, SN-1 dan E-2 SN-2, SN-1 SN-2, SN-1 7. Vinil Halida 8. Aril Halida Dalam Pembahasan Tersendiri 3 Organic compounds with an electronegative atom or an electron-withdrawing group bonded to a sp3 carbon undergo substitution or elimination reactions Halide ions are good leaving groups. Substitution reaction on these compounds are easy and are used to get a wide variety of compounds alkyl fluoride - alkyl chloride alkyl bromide alkyl iodide 4 Alkyl Halides in Nature Synthesized by red algae red algae Synthesized by sea hare a sea hare 5 Substitution Reaction with Halides (1) bromomethane If concentration of (1) is doubled, the rate of the reaction is doubled. If concentration of (2) is doubled, the rate of the reaction is doubled. (2) methanol If concentration of (1) and (2) is doubled, the rate of the reaction quadruples. 6 Substitution Reaction with Halides (1) (2) bromomethane methanol Rate law: rate = k [bromoethane][OH-] this reaction is an example of a SN2 reaction. S stands for substitution N stands for nucleophilic 2 stands for bimolecular 7 Mechanism of SN2 Reactions Alkyl halide The rate of reaction depends on the concentrations of both reactants. When the hydrogens of bromomethane are replaced with methyl groups the reaction rate slow down. The reaction of an alkyl halide in which the halogen is bonded to an asymetric center leads to the formation of only one stereoisomer Relative rate 1200 40 1 ≈0 8 Mechanism of SN2 Reactions Hughes and Ingold proposed the following mechanism: Transition state Increasing the concentration of either of the reactant makes their collision more probable. 9 Mechanism of SN2 Reactions Steric effect Energy activation energy: DG2 activation energy: DG1 reaction coordinate reaction coordinate Inversion of configuration (S)-2-bromobutane (R)-2-butanol 10 Factor Affecting SN2 Reactions The leaving group - + RCH I 2 HO HO HO HO + RCH2Br + RCH2Cl + RCH2F - RCH2OH + I RCH2OH + Br RCH2OH + Cl RCH2OH + F relative rates of reaction 30 000 10 000 200 1 pKa HX -10 -9 -7 3.2 The nucleophile In general, for halogen substitution the strongest the base the better the nucleophile. pKa Nuclephilicity 11 SN2 Reactions With Alkyl Halides an alcohol a thiol an ether a thioether an amine an alkyne a nitrile 12 Substitution Reactions With Halides 1-bromo-1,1-dimethylethane 1,1-dimethylethanol Rate law: If concentration of (1) is doubled, the rate of the reaction is doubled. If concentration of (2) is doubled, the rate of the reaction is not doubled. rate = k [1-bromo-1,1-dimethylethane] this reaction is an example of a SN1 reaction. S stands for substitution N stands for nucleophilic 1 stands for unimolecular 13 Mechanism of SN1 Reactions Alkyl halide Relative rate The rate of reaction depends on the concentrations of the alkyl halide only. ≈0* When the methyl groups of 1-bromo1,1-dimethylethane are replaced with hydrogens the reaction rate slow down. ≈0* The reaction of an alkyl halide in which the halogen is bonded to an asymetric center leads to the formation of two stereoisomers 12 1 200 000 * a small rate is actually observed as a result of a SN2 14 Mechanism of SN1 Reactions nucleophile attacks the carbocation slow C-Br bond breaks fast Proton dissociation 15 Mechanism of SN1 Reactions Rate determining step DG Carbocation intermediate R++ X+ R-OH2 R-OH 16 Mechanism of SN1 Reactions Inverted configuration relative the alkyl halide Same configuration as the alkyl halide 17 Factor Affecting SN1 reaction Two factors affect the rate of a SN1 reaction: • The ease with which the leaving group dissociate from the carbon • The stability of the carbocation The more the substituted the carbocation is, the more stable it is and therefore the easier it is to form. As in the case of SN2, the weaker base is the leaving group, the less tightly it is bonded to the carbon and the easier it is to break the bond The reactivity of the nucleophile has no effect on the rate of a SN1 reaction 18 Comparison SN1 – SN2 SN1 SN2 A two-step mechanism A one-step mechanism A unimolecular rate-determining step A bimolecular rate-determining step Products have both retained and inverted configuration relative to the reactant Product has inverted configuration relative to the reactant Reactivity order: 3o > 2o > 1o > methyl Reactivity order: methyl > 1o > 2o > 3o 19 Kestabilan Karbokation H H + H H H propan-2-ylium H H +H C 2 H H CH3+ H Ethanylium Methanylium 20 21 Elimination Reactions 1-bromo-1,1-dimethylethane 2-methylpropene Rate law: rate = k [1-bromo-1,1-dimethylethane][OH-] this reaction is an example of a E2 reaction. E stands for elimination 2 stands for bimolecular 22 The E2 Reaction A proton is removed Br- is eliminated The mechanism shows that an E2 reaction is a one-step reaction 23 Elimination Reactions 1-bromo-1,1-dimethylethane 2-methylpropene Rate law: If concentration of (1) is doubled, the rate of the reaction is doubled. rate = k [1-bromo-1,1-dimethylethane] If concentration of (2) is doubled, the rate of the reaction is not doubled. this reaction is an example of a E1 reaction. E stands for elimination 1 stands for unimolecular 24 The E1 Reaction The base removes a proton The alkyl halide dissociate, forming a carbocation The mechanism shows that an E1 reaction is a two-step reaction 25 Products of Elimination Reaction 30% 2-bromobutane 50% 80% 2-butene 20% 1-butene The most stable alkene is the major product of the reaction for both E1 and E2 reaction For both E1 and E2 reactions, tertiary alkyl halides are the most reactive and primary alkyl halides are the least reactive The greater the number of alkyl substituent the more stable is the alkene 26 ELIMINATION REACTIONS: ALKENES, ALKYNES 27 Elimination Reactions Dehydrohalogenation (-HX) and Dehydration (-H2O) are the main types of elimination reactions. C C C C + X Y X Y 28 Dehydrohalogenation (-HX) H C C strong C C + "H X" base X X = Cl, Br, I 29 The E2 mechanism This reaction is done in strong base at high concentration, such as 1 M NaOH in water. H_ H .. __ O: .. .. O: H H C + C C C Br _ + Br concerted mechanism 30 Kinetics • The reaction in strong base at high concentration is second order (bimolecular): Rate law: rate = k[OH-]1[R-Br]1 31 The E1 mechanism This reaction is done in strong base such as 0.01 M NaOH in water!! Actually, the base solution is weak! 1) H C H slow C rate determining step Br H 2) H C C + _ Br + H .. O: fast H C C + .. + O H H + C C 32 Kinetics • The reaction in weak base or under neutral conditions will be first order (unimolecular): • Rate law: rate = k [R-Br]1 • The first step (slow step) is rate determining! 33 The E2 mechanism • • • • • • Mechanism Kinetics Stereochemistry of reactants Orientation of elimination (Zaitsev’s rule) Stereochemistry of products Competing reactions 34 E2 mechanism This reaction is done in strong base at high concentration, such as 1 M NaOH in water. H H .. __ O: .. .. O: H H C + C C C Br _ + Br concerted mechanism 35 Kinetics of an E2 reaction • The reactions are second order (bimolecular reactions). • Rate = k [R-Br]1[Base]1 second order reaction (1 + 1 = 2) High powered math!! 36 Transition State H .. dO: H C C Br d- energy H .. __ O: .. H O H C C Br C H C Br- Reaction coordinate 37 Stereochemistry of reactants • E2 reactions must go by an anti elimination • This means that the hydrogen atom and halogen atom must be 180o (coplanar) with respect to each other!! • Draw a Newman projection formula and place the H and X on opposite sides. 38 Stereochemistry of E2 Reaction CH3 C CH3 CH3 Br CH3 H H H H KOH Alcohol Solvent CH3 C CH3 H H H H and Br are anti structure in conformation!!!!!!!!! 39 (S,S)-diastereomer CH3 Br CH3 C H t-butyl C H KOH ethanol heat ??? CH3 CH3 C H ??? H C CH3 C t-butyl (E)-isomer CH3 C t-butyl (Z)-isomer 40 This one is formed! CH3 CH3 C H C T-butyl (E)-isomer 41 (R,S)-diastereomer CH3 Br C H CH3 t-butyl C H KOH ethanol heat ??? CH3 CH3 C H ??? H C CH3 C T-butyl (E)-isomer CH3 C t-butyl (Z)-isomer 42 This one is formed! H CH3 C CH3 C t-butyl (Z)-isomer 43 Orientation of elimination: regiochemistry/ Zaitsev’s Rule • In reactions of removal of hydrogen halides from alkyl halides or the removal of water from alcohols, the hydrogen which is lost will come from the more highly-branched b-carbon. More branched H H H b H C C C CH3 H X H b C H Less branched A. N. Zaitsev -- 1875 H 44 Product formed from previous slide H H C H H C CH3 H C C H H More substituted alkene is more stable!!!!!!!! 45 Typical bases used in E2 reactions High concentration of the following >1M If the concentration isn’t given, assume that it is high concentration! • Na+ -OH • K+ -OH • Na+ -OR • Na+ -NH2 46 Orientation of elimination: regiochemistry/ Zaitsev’s Rule Explaination of Zaitsev’s rule: When you remove a hydrogen atom from the more branched position, you are forming a more highly substituted alkene. 47 Stereochemistry of products • The H and X must be anti with respect to each other in an E2 reaction! • You take what you get, especially with diastereomers! See the previous slides of the reaction of diastereomers. 48 Competing reactions • The substitution reaction (SN2) competes with the elimination reaction (E2). • Both reactions follow second order kinetics! 49 The E1 mechanism • Mechanism • Kinetics • Stereochemistry of reactants • Orientation of elimination (Zaitsev’s rule) • Stereochemistry of products • Competing reactions 50 E1 mechanism This reaction is done in strong base at low concentration, such as 0.01 M NaOH in water) 1) H C H slow C water helps to stabilize carbocation Br H 2) H C C + H .. O: H fast H C C + _ Br + ..+ O H + C C 51 E1 Reactions • These reactions proceed under neutral conditions where a polar solvent helps to stabilize the carbocation intermediate. • This solvent also acts as a weak base and removes a proton in the fast step. • These types of reactions are referred to as solvolysis reactions. 52 • tertiary substrates go by E1 in polar solvents, with little or no base present! • typical polar solvents are water, ethanol, methanol and acetic acid • These polar solvents help stabilize carbocations • E1 reactions also occur in a low concentration of base (i.e. 0.01M NaOH). 53 However!!!! •With strong base (i.e. >1M), goes by E2 54 Structure of the Carbocation Intermediate CH3 C CH3 CH3 55 Carbocation stability order Tertiary (3o) > secondary (2o) > primary (1o) It is hard (but not impossible) to get primary compounds to go by E1. The reason for this is that primary carbocations are not stable! 56 Kinetics of an E1 reaction • E1 reactions follow first order (unimolecular) kinetics: Rate = k [R-X]1 • The solvent helps to stabilize the carbocation, but it doesn’t appear in the rate law!! 57 dBr d+ C C H d+ C H C d+ + C C energy H intermediate Br C C H C C + H+ Reaction coordinate 58 Stereochemistry of the reactants • E1 reactions do not require an anti coplanar orientation of H and X. • Diastereomers give the same products with E1 reactions, including cis- and trans products. • Remember, E2 reactions usually give different products with diastereomers. 59 Orientation of elimination • E1 reactions faithfully follow Zaitsev’s rule! • This means that the major product should be the product that is the most highly substituted. 60 Stereochemistry of products E1 reactions usually give the thermodynamically most stable product as the major product. This usually means that the largest groups should be on opposite sides of the double bond. Usually this means that the trans product is obtained. 61 Competing reactions • The substitution reaction (SN1) competes with the elimination reaction (E1). • Both reactions follow first order kinetics! 62 Whenever there are carbocations… • They can undergo elimination (E1) • They can undergo substitution (SN1) • They can rearrange – and then undergo elimination – or substituion 63 Rearrangements • Alkyl groups and hydrogen can migrate in rearrangement reactions to give more stable intermediate carbocations. • You shouldn’t assume that rearrangements always occur in all E1 reactions, otherwise paranoia will set in!! 64 Comparison of E2 / E1 • E1 reactions occur under essentially neutral conditions with polar solvents, such as water, ethyl alcohol or acetic acid. • E1 reactions can also occur with strong bases, but only at low concentration, about 0.01 to 0.1 M or below. • E2 reactions require strong base in high concentration, about 1 M or above. 65 Comparison of E2 / E1 • E1 is a stepwise mechanism (two or more); Carbocation intermediate! • E2 is a concerted mechanism (one step) No intermediate! • E1 reactions may give rearranged products • E2 reactions don’t give rearrangement • Alcohol dehydration reactions are E1 66 Bulky leaving groups Hofmann Elimination This give the anti-Zaitsev product (least substituted product is formed)! b b _ CH3 CH2 CH2 CH CH3 OH CH N+ CH 3 heat CH3 CH2 CH CH CH3 6% 3 CH3 + CH3 CH2 CH2 CH CH2 94% 67 Orientation of elimination: regiochemistry/ Hofmann’s Rule • In bimolecular elimination reactions in the presence of either a bulky leaving group or a bulky base, the hydrogen that is lost will come from the LEAST highly-branched b-carbon. More branched H H H b H C C C CH3 H X Less branched H b C H H 68 Product from previous slide H H C H H C CH3 H C C H H 69 Elimination with bulky bases • Non-bulky bases, such as hydroxide and ethoxide, give Zaitsev products. • Bulky bases, such as potassium tertbutoxide, give larger amounts of the least substituted alkene (Hoffmann) than with simple bases. 70 Comparing Ordinary and Bulky Bases H CH3 C CH CH3 NaOC2H5 C2H5OH heat CH3 Br H CH3 C CH3 C CH CH3 Major CH CH2 Major CH3 H CH CH3 Br CH3 KOC(CH3)3 (CH3)3COH heat CH3 C CH3 71 1-butene: watch out for competing reactions! H3C CH2 CH2 CH2 O-CH3 Non-bulky KOCH 3 H3C CH2 CH2 CH2 SN2 Br bulky base KO-t-butyl E2 H3C CH2 CH CH2 72 Highlights • • • • • Dehydrohalogenation -- E2 Mechanism Zaitsev’s Rule Dehydrohalogenation -- E1 Mechanism Carbocation Rearrangements -- E1 Elimination with Bulky Leaving Groups and Bulky Bases -- Hofmann Rule -- E2 73 Competition Between SN2/E2 and SN1/E1 SN1 SN2 E1 E2 rate = k1[alkyl halide] + k2[alkyl halide][nucleo.] + k3[alkyl halide] + k2[alkyl halide][base] • SN2 and E2 are favoured by a high concentration of a good nucleophile/strong base • SN1 and E1 are favoured by a poor nucleophile/weak base, because a poor nucleophile/weak base disfavours SN2 and E2 reactions 74 Competition Between Substitution and Elimination • SN2/E2 conditions: In a SN2 reaction: 1o > 2o > 3o In a E2 reaction: 3o > 2o > 1o 10% 90% 75% 25% 100% 75 Competition Between Substitution and Elimination • SN1/E1 conditions: All alkyl halides that react under SN1/E1 conditions will give both substitution and elimination products (≈50%/50%) 76 Summary • Alkyl halides undergo two kinds of nucleophilic subtitutions: SN1 and SN2, and two kinds of elimination: E1 and E2. • SN2 and E2 are bimolecular one-step reactions • SN1 and E1 are unimolecular two step reactions • SN1 lead to a mixture of stereoisomers • SN2 inverts the configuration od an asymmetric carbon • The major product of a elimination is the most stable alkene • SN2 are E2 are favoured by strong nucleophile/strong base • SN2 reactions are favoured by primary alkyl halides • E2 reactions are favoured by tertiary alkyl halides 77 REAKSI ADISI ALKENA 78 Addition Reaction of Alkene 1. HX Addition • Electrophilic Addition (Markovnikov Product) • Free Radical Mechanism (Anti-Mark Product) 2. Hydration (+ H2O) 3. Halogenation/ Hydrohalogenation 4. Reduction or Hydrogenation (+ H2 ) 5. Oxidation 6. Multi-step Synthesis Addition of Halogens to Alkenes C C + X2 X C C • electrophilic addition to double bond • forms a vicinal dihalide X2 = Cl2 or Br2 F2; explosive I2 ; endothermic X Example Br2 CH3CH CHCH(CH3)2 CH3CHCHCH(CH3)2 Br Br (100%) Stereochemistry of Halogen Addition •anti addition H Br2 H Br Br H H trans-1,2-Dibromocyclopentane 80% yield; only product Anti Addition ; Two Bromines add to opposite sides of the ring Example H H Cl Cl2 H H Cl trans-1,2-Dichlorocyclooctane 73% yield; only product Mechanism is electrophilic addition •Br2 is not polar, but it is polarizable •two steps (1) formation of bromonium ion & • • electrophilic attack (2) nucleophilic attack on bromo ion by bromide NET REACTION CH2=CH2 + Br2 -> Br-CH2-CH2-Br Step 1a: Formation of Bromonium Ion Br Br d– Br Br d+ Mutual polarization of electron distributions of Br2 and alkene Electrons flow from alkene toward Br2 d+ Step 1b; Electrophilic Addition to form Bromonium Ion Part i H H C H C + Br Br H Br H C + C H H + Br- H Part ii Step 1b; Lone Pair on Bromine Stabalizes Carbocation and Forms Cyclic Bromonium Ion H C + Br Br + C H H C C H + Br- H H H + Br H Step 2; Bromide Ion Must Attack from Oppositte Side of Cyclic Bromonium Ion (anti addition) + Br Br C C H H H + H H Br C C Br H H + Br H Br Example H Br2 H H Br Br H trans-1,2-Dibromocyclopentane 80% yield; only product C C + X2 C X C X alkenes react with X2 to form vicinal dihalides alkenes react with X2 in water to give vicinal halohydrins C C + X2 + H2O X C C + H—X OH Examples H2C CH2 + H2O Br2 BrCH2CH2OH (70%) H Cl2 H OH H2O H Cl H anti addition: only product Mechanism; 1) Cl2 is polarized and adds across double bond. 2) Ion formed is stabalized by lone pair of Cl. 3) Water attacks chloronium ion from side opposite (anti addition) carbonchlorine bond. This gives trans isomer Regioselectivity CH3 H3C Br2 C CH3 CH2 C CH2Br H2O H3C OH (77%) •Markovnikov's rule applied to halohydrin formation: the halogen adds to the carbon having the greater number of hydrogens. Hydrogenation (Reduction, +H2) of Ethylene H C H H Metal Catalyst C + H H—H H H H C C H H •exothermic DH° = –136 kJ/mol •catalyzed by finely divided Pt, Pd, Rh, Ni H Two spatial (stereochemical) aspects of alkene hydrogenation: •(1) syn addition of both H atoms to double bond •(2) hydrogenation is stereoselective, corresponding to addition to less crowded face of double bond H CO2CH3 CO2CH3 H2, Pt CO2CH3 CO2CH3 H syn-Additon versus antiAddition syn addition anti addition syn-Addition; Metal catalyst breaks H-H bonds. B Y C C A H X H H H syn-Addition; Addition of H2 across double bonds takes place in two steps. B Y A X H H C H C H H3C Example of Stereoselect ive Reaction CH3 H H3C H3C H2, cat H3C CH3 H H H H3C H Both products correspond to syn addition of H2. H H H3C CH3 H3C CH3 H H3C H3C H2, cat Top face of double bond blocked by this methyl group CH3 H H H3C H But only this one is formed. H2 adds to bottom face of double bond. Example of Stereoselect ive Reaction