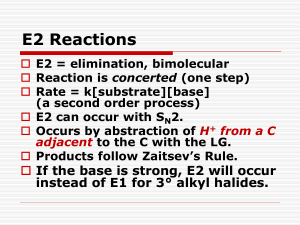
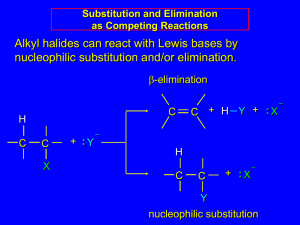
Alkyl Halides
advertisement

ORGANIC CHEMISTRY CHM 207 CHAPTER 5: ALKYL HALIDES NOR AKMALAZURA JANI SUBTOPICS • Nomenclature and structures of alkyl halides. • Classification of alkyl halides. • Physical properties of alkyl halides. • Reaction of alkyl halides. 1) Formation of alkanes (Wurtz reaction) 2) Nucleophilic substitution reaction: i) Example: formation of alcohol, Williamson ether synthesis, amine synthesis, nitrile synthesis ii) Mechanism of nucleophilic substitution reactions. iii)Types of nucleophilic substitution reaction: SN1 and SN2 reaction. 3) Elimination reaction (dehydrohalogenation of alkyl halides). - E1 and E2 reactions. • Uses of alkyl halides. ALKYL HALIDES • General formula: CnH2n+1X where n = 1,2,… and X (halogen) • Functional group: halogen, -X (X = F, Cl, Br, I) • Naming alkyl halides: - same as nomenclature of alkanes 5 CH3 I iodomethane 4 3 CH3 2 CH3 1 CH3 CH2 CH CH CH3 CI 3-chloro-2-methylpentane 6 5 4 CH3 CH2 C CH3 3 2 1 CH2 CH CH3 Br 4-bromo-2,4-dimethylhexane no. of alkyl groups: 2 H R' C X R Classification of alkyl halides a) Primary (1 ) c) Tertiary (3o) no. of alkyl groups: none or one no. of alkyl groups: 3 o H H C X H H H C X R o b) Secondary (2 ) no. of alkyl groups: 2 H R' C X R c) tertiary (3o) no. of alkyl groups: 3 R'' R' C X R PHYSICAL PROPERTIES • BOILING POINTS - molecules with higher molecular weight have higher boiling points. - reasons: the molecule is heavier, slower moving, have greater surface area, have larger London attractions, resulting higher boiling points. - example: RMM bp (°C) CH3F 34 -78 CH3Cl 50.5 -24 CH3Br 95 4 CH3I 142 42 - compounds with branched have more spherical shapes, have smaller surface area, resulting lower boiling points. CH3 CH3 CH3CH2CH2CH2Cl bp 78 oC CH3 C Cl CH3CH2CHCl CH3 bp 67 oC o bp 52 C - alkyl halides with more carbon atoms have higher boiling points. CH3Cl o bp -24 C CH3CH2Cl bp 12oC CH3CH2CH2Cl o bp 47 C • DENSITIES - alkyl fluoride and alkyl chlorides (with one Cl atom) are less dense than water. - alkyl chloride with two or more chlorine atoms are denser than water. - all alkyl bromides and alkyl iodides are denser than water. REACTIONS OF ALKYL HALIDES Formation of alkanes (Wurtz reaction) • Equation: 2R-X + 2Na → 2NaX + R-R • Example: 2CH3I + 2Na dry ether CH3-CH3 + 2NaI reflux • Most suitable for preparation of higher alkanes containing an even number of carbon atoms. • Alkanes containing an odd number of carbon atoms can be prepared by using a mixture of two different alkyl halides. • This reaction produces only low yields due to the formation of other alkanes as byproducts. CH3I + 2Na + CH3CH2I dry ether CH3CH2CH3 + 2NaI + CH3CH2-CH2CH3 + CH3CH3 by-products Reactions of alkyl halides • Two types of reactions: i) substitution reactions ii) elimination reactions a) nucleophilic substitution C C Nuc H X - X C C - H Nuc b) elimination B - C C H X B-H C C X - NUCLEOPHILIC SUBSTITUTION REACTIONS 1) Formation of alcohol OH R-X R-OH X nucleophile example CH3CH2 Br CH3CH2 OH NaOH ethyl bromide NaBr ethyl alcohol 2) Williamson ether synthesis R-X R'O R-O-R' X nucleophile example CH3 I CH3CH2 O Na+ methyl iodide CH3CH2 OH sodium ethoxide Na CH3 O CH2CH3 ethyl methyl ether CH3CH2 O Na+ sodium ethoxide Na+ I- 3) Amine synthesis R-X NH3 nucleophile example CH3CH2 Br H-NH2 ethyl bromide R-NH2 HX CH3CH2 NH2 HBr ethylamine (primary amine) HBr(g) + NH3 (g) NH4Br (s) amine are also act as nucleophile (more reactive than ammonia) C2H5Br H N C2H5 H C2H5Br (C2H5)2NH (C2H5)2NH + HBr diethylamine (secondary amine) (C2H5)3N + HBr triethylamine (tertiary amine) C2H5Br (C2H5)3N (C2H5)4N+ Brtetraethylammonium bromide (quaternary salt) 4) Nitrile synthesis R-X CN cyanide (nucleophile) H2O/H+ R-CN H2/Ni 180oC X R-CN nitrile R-COOH (hydrolysis) R-CH2NH2 (reduction) example (CH3)2CHCH2CH2-Cl NaCN 4-methylpentanenitrile 1-chloro-3-methylbutane H2O/H+ (CH3)2CHCH2CH2-CN (CH3)2CHCH2CH2-CN H2/Ni 180oC (CH3)2CHCH2CH2-COOH (CH3)2CHCH2CH2-CH2NH2 NaCl Mechanism of nucleophilic substitution reactions H δδ+ R C X Nu - H H R C Nu X - H EXAMPLE H OHδ+ δCH3 C Br H formation of alcohol CH3 H C OH H - Br Type of nucleophilic substitution reactions: SN1 and SN2 reactions • • • • S = substitution N = nucleophilic 1 = a first order (unimolecular) reaction 2 = a second order (bimolecular) reaction SN1 (Substitution, Nucleophilic, unimolecular) reactions • Unimolecular : only one molecule involved in the transition state of the rate-limiting step. • Example: the reaction between aqueous NaOH and tertiary alkyl halides. (CH3)3C-Br + OH- slow (CH3)3C-OH + Br- MECHANISM OF SN1 REACTION STEP 1: FORMATION OF CARBOCATION (CH3)3C Br slow (CH3)3C+ + Br very reactive STEP 2: NUCLEOPHILIC ATTACK (CH3)3C+ + OH- fast (CH3)3C-OH - rate limiting step • The reaction is first order and the rate depends only on the concentration of the tertiary alkyl halides. Rate = k[(CH3)3CBr] • The concentration of OH- does not have any effect on the rate of reaction. • OH- does not involved in the rate-limiting step. Carbocation rearrangement in SN1 reactions • Rearrangement of the carbon skeleton will take place if a more stable carbocation can be formed in the process. • For example, hydrolysis of the secondary alkyl bromide, 2bromo-3-methylbutane, yields the tertiary alcohol, 2-methyl-2butanol. CH3 H CH3 H CH3 C C H Br CH3 SN1 CH3 H2O C o C CH3 Br- H slow step (2 carbocation) 2-bromo-3-methylbutane shift of H CH3 H CH3 C C CH3 H CH3 H o (3 carbocation) H-OH fast CH3 C C CH3 OH H 2-methyl-2-butanol • Reactivity towards SN1 substitution mechanisms follows the stability of carbocations: SN1 reactivity: 3o > 2o > 1o > CH3X retention inversion SN2 (Substitution, Nucleophilic, bimolecular) reactions • The processes of bond breaking and bond forming occur simultaneously (one bond is forming, one bond is breaking). • The mechanism involves only one step. • For example, hydrolysis of iodomethane (primary alkyl halides) HO H δ+ δC I H H HO C iodomethane transition state H δ- H δ+ δ- I H H - I HO C H methanol H or HO CH3 I iodomethane HO CH3 I transition state HOCH3 methanol - I rate-limiting step • A second order reaction • Rate equation = k[CH3I][OH-] • Both iodomethane and the OH- are involved in the rate-limiting step. • SN2 reactivity: CH3 X > 1o > 2o > neopentyl > 3o • Factor that determines the order of reactivity in SN2 reactions is the steric effect. • A steric effect is one in which the rate of chemical reaction depends on the size or spatial arrangement of the groups attached to, or near to, the reaction site of the molecule. Relative reactivities of primary, secondary, and tertiary alkyl halides • The reactivity of alkyl halides towards nucleophilic substitution depend on the halogen. • The rate of reaction decrease in the order R-I > R-Br > R-Cl > R-F (most reactive) (least reactive) • Reason: C-X bond become stronger from I to F Comparison SN1 and SN2 reactions SN1 Rate of reaction First order SN2 Second order Stereochemistry Racemic mixture Complete (mixture of inversion inversion and retention) Reactivity Benzyl > allyl > ~ 3o CH3X > 1o > 2o > 3o > 2o > 1o Nucleophiles Weak nucleophiles Strong nucleophiles Elimination reactionsdehydrohalogenation of alkyl halides • Elimination: loss of two atoms or groups from the substrate to form a pi bonds. • Dehydrohalogenation (removal of hydrogen and a halogen atom) of alkyl halide to form alkene. C C H X alkyl halide HX = HCl or HBr or Hl C C alkene HX • The elimination reaction is occurred when the reaction used strong base for examples, t-butoxide ion ((CH3)3CO-) or OH- ion and heated at high temperature. • Dehydrohalogenation will yield an alkene that has the larger number of alkyl groups as the main product (Saytzeff’s rule). • Elimination reactions can be divided into two: i) E1 reaction ii) E2 reaction E1(Elimination, unimolecular) reaction • The rate-limiting state involves a single molecular than a collision between two molecules. • A first order reaction. • Rate equation: k[RX] • E1 reactivity: Benzyl > allyl > 3o > 2o > 1o MECHANISM OF E1 REACTION STEP 1: FORMATION OF THE CARBOCATION (RATE LIMITING) C C C H X H C X STEP 2: A BASE ABSTRACTS A PROTON (FAST) B C H C B-H C C E2(Elimination, bimolecular) reaction • • • • A second order reaction. Rate equation: k[RX][Base] E2 reactivity: 3o > 2o > 1o Mechanism: B H C C C X C B-H X- Comparison E1 and E2 reactions E1 Rate of reaction First order E2 Second order Reactivity 3o > 2o > 1o 3o > 2o > 1o Base Do not need strong base Strong base Alkyl halides 1O Reactions RCH2X 2O NuSN2 RCH2Nu Nu- strong SN2 + E2 R2CHNu + alkene R2CHX B- (strong) alkene E2 Nu- (weak) SN1 + E1 3O R3CNu + alkene R3CX B- (strong) E2 alkene SOME COMMON NUCLEOPHILES strong nucleophiles moderate nucleophiles weak nucleophiles (CH3CH2)3P S-H I (CH3CH2)2NH C N (CH3CH2)2N H-O CH3-O Br NH3 F H-O-H CH3-S-CH3 Cl O CH3C O CH3 O H USES OF ALKYL HALIDES • Solvents - industrial and household solvents. - carbon tetrachloride (CCl4) used for dry cleaning, spot removing. - methylene chloride (CH2Cl2) is used to dissolve the caffeine from coffee beans to produce decaffeinated coffee. • Reagents - as starting materials for making complex molecules. - for example, the conversion of alkyl halides to organometallic reagents (compounds containing carbonmetal bonds) is important tool for organic synthesis. • Anesthetics - examples: chloroform (CHCl3) and ethyl chloride. • Freons: Refrigerants and foaming agents - Freons (called chlorofluorocarbons, or CFCs) is used as a refrigerant gas. • Pesticides - example: DDT (Dichloro DiphenylTrichloroethane) is used as insecticides.