Alkanes
advertisement

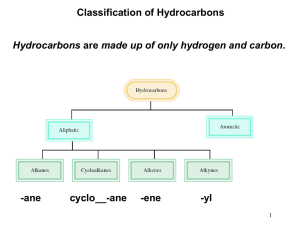
Alkanes Hydrocarbons Aliphatic Aromatic Hydrocarbons Aliphatic Alkanes Aromatic Alkenes Alkynes Hydrocarbons Aliphatic Alkanes Alkanes are hydrocarbons in which all of the bonds are single bonds. H H H C C H H H Hydrocarbons Aliphatic Alkenes are hydrocarbons that contain a carboncarbon double bond. H H Alkenes C H C H Hydrocarbons Aliphatic Alkynes are hydrocarbons that contain a carboncarbon triple bond. Alkynes HC CH Hydrocarbons The most common aromatic hydrocarbons are those that contain a benzene ring. Aromatic H H H H H H CnH2n+2 Introduction to Alkanes: Methane, Ethane, and Propane The Simplest Alkanes Methane (CH4) CH4 Ethane (C2H6) CH3CH3 Propane (C3H8) CH3CH2CH3 bp -160°C bp -89°C bp -42°C Structure of Methane tetrahedral bond angles = 109.5° bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Structure of Ethane C2H6 CH3CH3 tetrahedral geometry at each carbon C—H bond distance = 110 pm C—C bond distance = 153 pm n-Butane CH3CH2CH2CH3 Isobutane (CH3)3CH bp -0.4°C bp -10.2°C C5H12 CH3CH2CH2CH2CH3 (CH3)2CHCH2CH3 n-Pentane Isopentane (CH3)4C Neopentane Number of Constitutionally Isomeric Alkanes CH4 1 C2H6 1 C3H8 1 C4H10 2 C5H12 3 C6H14 5 C7H16 9 C8H18 18 C9H20 35 C10H22 75 C15H32 4,347 C20H42 366,319 C40H82 62,491,178,805,831 Nomenclature of Alkanes Rules for naming compounds are given by the International Union for Pure and Applied Chemistry (IUPAC). To name alkanes: Find the longest chain and use it as the name of the compound. Number the carbon atoms starting with the end closest to the substituent. Name and give the location of each substituent. When two or more substituents are present list them in alphabetical order. Nomenclature of Alkanes 1. Determine the number of carbons in the parent hydrocarbon 8 7 6 5 4 3 2 8 1 CH3CH2CH2CH2CHCH2CH2CH3 7 6 5 4 4 CH3CH2CH2CH2CHCH2CH3 CH3 2 2 1 CH3CH2CH2CHCH2CH2CH3 CH2CH2CH3 3 3 CH2CH2CH2CH3 1 5 6 7 8 2. Number the chain so that the substituent gets the lowest possible number 1 2 3 4 5 CH3CHCH2CH2CH3 CH3 2-methylpentane 1 2 3 4 5 6 7 8 CH3CH2CH2CHCH2CH2CH2CH3 CHCH3 CH3 4-isopropyloctane CH3 CH3CHCH2CH2CH3 common name: isohexane systematic name: 2-methylpentane 3. Number the substituents to yield the lowest possible number in the number of the compound CH3CH2CHCH2CHCH2CH2CH3 CH3 CH2CH3 5-ehtyl-3-methyloctane not 4-ethyl-6-methyloctane because 3<4 (substituents are listed in alphabetical order) 4. Assign the lowest possible numbers to all of the substituents CH3 CH3 CH3CH2CHCH2CHCH3 CH3 CH3 2,4-dimethylhexane CH3CH2CH2C CCH2CH3 CH3 CH3 3,3,4,4-tetramethylheptane CH2CH3 CH3 CH3CH2CHCH2CH2CHCHCH2CH2CH3 CH2CH3 CH2CH3 3,3,6-triethyl-7-methyldecane 5. When both directions lead to the same lowest number for one of the substituents, the direction is chosen that gives the lowest possible number to one of the remaining substituents CH3 CH3 CH3CHCH2CHCH3 CH3 CH3 2,2,4-trimethylpentane not 2,4,4-trimethylpentane because 2<4 CH2CH3 CH3CH2CHCHCH2CHCH2CH3 CH3 6-ethyl-3,4-dimethyloctane not 3-ethyl-5,6-dimethyloctane because 4<5 6. If the same number is obtained in both directions, the first group receives the lowest number CH2CH3 Cl CH3CH2CHCH3 Br 2-bromo-3-chlorobutane not 3-bromo-2-chlorobutane CH3CH2CHCH2CHCH2CH3 CH3 3-ethyl-5-methylheptane not 5-ethyl-3-methylheptane 7. In the case of two hydrocarbon chains with the same number of carbons, choose the one with the most substituents 3 4 5 6 CH3CH2CHCH2CH2CH3 2 CHCH3 1 CH3 3-ethyl-2-methylhexane (two substituents) 1 2 3 4 5 6 CH3CH2CHCH2CH2CH3 CHCH3 CH3 3-isopropylhexane (one substituent) 8. Certain common nomenclatures are used in the IUPAC system CH3CH2CH2CH2CHCH2CH2CH3 CHCH3 CH3 4-isopropyloctane or 4-(1-methylethyl)octane CH3CH2CH2CH2CHCH2CH2CH2CH2CH3 CH2CHCH3 CH3 5-isobutyldecane or 5-(2-methylpropyl)decane Nomenclature of Alkanes Types of Carbons primary (1o) - bonded to only 1 other carbon secondary (2o) - bonded to 2 other carbons tertiary (3o) - bonded to 3 other carbons quaternary (4o) - bonded to 4 other carbons CH3 CH3 CH3 C CH2 CH CH3 CH3 Conformations are different spatial arrangements of a molecule that are generated by rotation about single bonds. Ethane Eclipsed conformation Ethane Staggered conformation Projection formulas of the staggered conformation of ethane H H H H H H H H H H H Newman H Sawhorse Anti relationships H H H H 180° H H H H H H H H Two bonds are anti when the angle between them is 180°. Gauche relationships H H H 60° H H H H H H H H H Two bonds are gauche when the angle between them is 60°. An important point: The terms anti and gauche apply only to bonds (or groups) on adjacent carbons, and only to staggered conformations. 12 kJ/mol 0° 60° 120° 180° 240° 300° 360° Conformational Analysis of Butane: C2-C3 Rotation The most stable conformation of unbranched alkanes has anti relationships between carbons Hexane Reactions with Alkanes The C-C and C-H bonds are very strong. Therefore, alkanes are very unreactive. At room temperature alkanes do not react with acids, bases, or strong oxidizing agents. Alkanes do combust in air (making them good fuels): 2C2H6(g) + 7O2(g) 4CO2(g) + 6H2O(l) H = -2855 kJ Alkanes are very unreactive compounds because they have only strong s bonds and atoms with no partial charges However, alkanes do react with Cl2 and Br2 Reaction of Alkane with Cl2 or Br2 Petroleum is a complex mixture of alkanes and cycloalkanes that can be separated by distillation