Learning Objective: HSW: to understand why we need food and to
advertisement

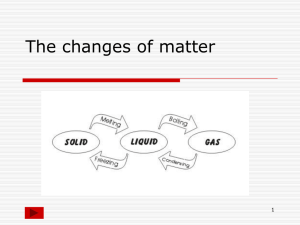
13 April, 2015 Kinetic Theory Objectives HSW: Use the kinetic theory to explain the different states of matter. Candidates should be able to draw simple diagrams to illustrate the difference between solids, liquids and gases. HSW: AF1 – Thinking Scientifically AF4 – Using investigative approaches Used before in: Will use again in: Lesson 2 – Energy Transformations PLTS: Independent enquirers: support conclusions, using reasoned arguments and evidence. Used before in: Will use again in: Keywords Boiling point, condense, evaporate, freezing point, melt, melting point, sublimation Learning Outcomes: All students should be able to: • Describe what happens at melting/freezing and boiling point • Label a general cooling curve. Most students should be able to: • Use the particle model to explain what is happening at each point on the cooling curve • Describe the difference between the temperatures of substances at boiling and evaporation point. Some students should be able to: Explain how evaporation makes a liquid cooler States of matter and temperature Water can be a solid, liquid or a gas At a cold enough temperature, even substances that are normally gases will become solid. At higher temperatures, solids change to become liquids or gases – as long as they do not catch fire or break down. Particles in a solid – animation Particles in a liquid – animation Particles in a gas – animation Changes of state Each change of state is given a different name: sublimation melting boiling liquid solid freezing gas condensing reverse sublimation Changes of state – heating curve boiling liquid gas melting condensing solid liquid freezing time Changes of state activity Changes of state – cooling curve activity What is evaporation? Evaporation occurs when the particles in a liquid escape to form a vapour. Evaporation can occur at any temperature but it occurs most rapidly at a liquid’s boiling point. The particles that escape take some energy from the remaining particles and so the temperature of the liquid falls. Evaporation is another method of heat transfer. Practical time AF1 – Thinking Scientifically AF4 – Using investigative approaches • Practical - Stearic Acid practical – follow method teacher has shown you. (method on next slide) • Draw your own cooling curve in your book and label each change of state • Now complete a “triangle/arrow summary diagram in books to show all changes of state and Particle diagrams of solids/liquids/gases Method • Take a boiling tube of molten wax from the water bath • Place in a boiling tube rack • Take the temperature of the wax every minute recording the state of matter. • Stop when the wax has been solid for 2 minutes with no temperature change • Draw a cooling curve from your results Plenary - Sublimation Sublimation, reverse sublimation OR: AB document link on page 127 opens a CLOZE exercise covering the material on page 126-127. There is also an AT presentation with the answers. Youtube video of sublimation of dry ice: http://www.youtube.com/watch?v=J8mDGwf -5x0