Development of Atomic Theory
advertisement

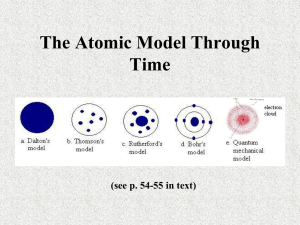
Development of Atomic Theory Physical Science Chapter 4 - Section1 The Beginnings • Atomic theory developed slowly over 2000 years • 4th century B.C., Greek philosopher Democritus suggested matter consists of indivisible units – Called these units “Atoms” – Atomos – Greek for indivisible • Not possible to acquire experimental evidence – No tools available during these ancient times Dalton’s Atomic Theory • English schoolteacher John Dalton used experimental observations in 1808 – Some portions are still true today • Concluded that all atoms of a given element are identical, and atoms of different elements join to form compounds Dalton’s Atomic Theory ( cont. ) • Observed different substances combining in proportions ( in consistent ways ) • Law of Definite Proportions • A given compound always has elements in exactly the same proportions by weight or mass • Water as example ( 11% H and 89% O ) • Atoms combine in whole number ratios ( H2O ) • Dalton’s theory did not fit all observations – With further experiments by others, some observations were found to be inaccurate J.J. Thompson’s Contribution • In 1897, J.J. Thompson accidently discovered electrons by experiment • Conducted experiments with Cathode Ray Tube – suggested cathode rays consist of negatively-charged particles that come from atoms – Cathode Ray Tube consists of two metal plates on either end of a vacuum tube ( Cathode – negative ; Anode – positive ) – Formed an electron beam ( by applying a voltage across two plates ) and observed that a magnet deflected ( bent ) the beam • His results also suggested that atoms could be divided into smaller parts J.J. Thompson’s Model • Suggested that atoms can be thought of as plum pudding ( alternatively, a blueberry muffin ) • In this model, electrons are spread throughout an atom ( mass and positive charge are evenly distributed throughout the atom however ) • Imagine a muffin with blueberries dispersed throughout it. – Blueberries are electrons – “Muffin” is the entire atom with the “bread” being the positive portion of the atom ( evenly distributed ) Earnest Rutherford • Another Englishmen during 19th-20th century • Developed experiment to test Thompson’s model • Found that Thompson’s model needed refining • In CONTRAST to Thompson’s model, Rutherford proposed that most of an atom’s mass was contained in its center ( the nucleus ) • Rutherford discovered the nucleus in a manner not vastly different than Thompson ( experiment ) Rutherford’s Experiment • Beam of positively charged alpha particles aimed at a very thin piece of gold foil • By Thompson’s Model the positive charge at any location in an atom would be too small to affect the paths of incoming particles • Rutherford predicted that most particles would remain straight and not be deflected – Again, assuming Thompson’s model is accurate – If the positive charge on an atom was concentrated, alpha articles would be deflected Rutherford’s Gold Foil Experiment • Results showed that the alpha particles WERE DEFLECTED • Some particles passed through, some were deflected by a large amount, some bounced straight back ( what did these hit? ) • This indicated: – That the positively-charged portion of the atom is NOT dispersed ( or IS concentrated in the atom ) – That Thompson’s model was not totally accurate – There is something in the middle of the atom!!! ( nucleus ) • Figure 7 ( pg. 118 ) shows model of atom after Rutherford’s work ( protons, neutrons, electrons )