mp - The University of Illinois Archives
advertisement

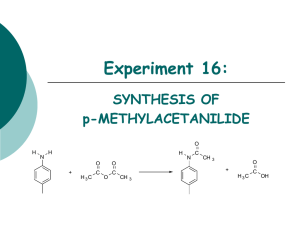
Melting Points and Mixed Melting Points Experiment 1: Identify a compound by its melting point and mixed melting points. Acetamide 113 - 115 oC p-Aminobenzoic acid 188 - 189 oC Camphoric Acid 183 - 186 oC trans-Cinnamic Acid 133 - 134 oC Malonic Acid 135 - 137 oC p-Nitrophenol 113 - 115 oC Resorcinol 110 - 113 oC Succinic Acid 187 - 189 oC Urea 133 - 135 oC Melting Point Tube A sample is put in the bottom of a melting point tube. Filling Tube Put a small amount of the compound in the open end of the melting point tube. Turn over and tape the closed end on the desk top until the compound falls to the bottom. Sample in the melting point tube. Thiele Tube Use a Thiele tube filled with mineral oil to heat your sample. Using Thiele Tube Attach the melting point tube to a thermometer. Heating Rate Heat about 5o per minute until within about 10o of the melting point. Near the melting point heat at 1 - 2o per minute Mel-Temp in the lab Temperature Starts to melt Finished melting m.p. = Start - Finish Salt on Roads Why is salt put on snow covered roads? Ice melts at What happens to the melting point if salt is added? Ice melts! o 0C Impurities Impurities such as salt lowers the melting point of water. Putting salt on icy roads causes the ice to melt because it lowers the melting point of water. Impure compounds usually melt lower than pure compounds so the melting point may be used as a measure of the purity of a compound Acetic Acid Acetic Acid, CH3COOH, is a colorless liquid that melts at 16.6oC. Let’s look at the melting point of mixtures of water and acetic acid. Eutectic Point Plot of melting point vs. mole fraction water for mixtures of water and acetic acid. Eutectic point Cool Cool a acetic acid - water solution with a mole fraction water of 0.9C Heat Heat a acetic acid - water solution with a mole fraction water of 0.9 Heat Heat a acetic acid - water solution with a mole fraction water of 0.2 Pure - Impure Pure compounds usually melt over a narrow temperature range, often 1o or less. Impure compounds melt lower than pure compounds and over a wider temperature range. Purity Melting points are a measure of purity m.p. = 115o - 119o m.p. = 118o - 120o m.p. = 121o - 122o Unknown Two of these bottles contain benzoic acid and one m-nitrophenylacetic acid. m.p. = 120o - 122o m.p. = 120o - 122o m.p. = 120o - 122o How do you tell what is in each bottle? Mixed Melting Points Grind samples together to be sure they are mixed and then measure the melting point. Results 1 2 3 Mixed 1 and 2 m.p. = 120o - 122o Mixed 1 and 3 m.p. = 114o - 117o Mixed 2 and 3 m.p. = 115o - 118o Summary 1. Measure the melting point of your unknown 2. Run mixed melting points to confirm identification