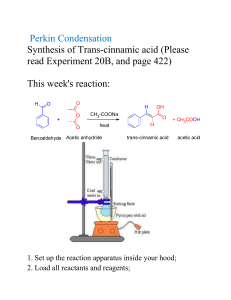
synthesis
advertisement

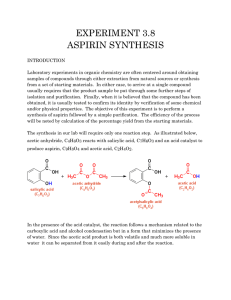
Experiment 16: SYNTHESIS OF p-METHYLACETANILIDE O H N H H + H 3C O O C C O N C CH 3 O + CH 3 H 3C C OH Objectives: To synthesize p-methylacetanilide from pmethylaniline and acetic anhydride. To isolate the product through a crystallization. To evaluate purity through melting point analysis. To analyze the product using IR and NMR spectra. Before coming to lab… Review these techniques: Suction filtration recrystallization SYNTHESIS OF THE AMIDE O H N H H + H 3C O O C C O N C CH 3 + H 3C CH 3 C 7H 9N C 4H 6O 3 C 9H 11N O M W :1 0 7 .1 5 g /m o l M W :1 0 2 .0 8 g /m o l M W :1 4 9 .1 9 g /m o l o m p : 4 1 -4 6 C O d :1 .0 8 g /m L o C OH C 2H 4O 2 M W :6 0 .0 5 g /m o l o m p : 1 4 8 -1 5 1 C d :1 .0 4 g /m L in g e stio n h a za rd bp: 140 C in g e stio n h a za rd b p : 1 1 7 -1 1 8 C in h a la tio n h a za rd in g e stio n h a za rd in h a la tio n h a za rd fla m m a b le in h a la tio n h a za rd fla m m a b le co rro siv e o co rro siv e MECHANISM O O C H O H N O + H 3C Nucleophilic nitrogen attacks electrophilic carbonyl of acetic anhydride… O H 3C C O H N H CH 3 The pi bond of the carbonyl reforms. A CO bond breaks, expelling the acetoxy group. CH 3 CH 3 …to form tetrahedral intermediate. CH 3 O O H C N CH 3 + H 3C C H N O C H + C H 3C O OH CH 3 CH 3 O CH 3 Loss of a proton to the acetoxy group produces the amide. SYNTHESIS Add solid amine and deionized water to 50 mL Erlenmeyer flask. Swirl to mix. Add acetic anhydride dropwise while swirling. React at room temp for 5 minutes, swirling occasionally. Dissolve solid on hotplate. Cool to room temp. Cool to 0o in ice water bath. PRODUCT ISOLATION Set up a suction filtration apparatus, clamping filter flask to ring stand. Attach red tubing to vacuum line. Weigh a small filter paper. Place it in the Buchner funnel and wet it with cold deionized water. Apply vacuum. Swirl flask and pour solid SLOWLY into center of funnel Leave under suction for several minutes. Transfer small filter paper containing product to a large preweighed filter paper. Submit product to instructor to dry until next lab period. Table 16.1 Theoretical yield (g) Actual yield (g) Cannot be obtained until following lab period since solid will not be dry. Cannot be calculated until following lab period when actual yield is obtained. % yield Melting range Can be calculated during experiment. (oC) Product Appearance Cannot be obtained until following lab period when solid is sufficiently dry. physical state and color of your product. Table 16.2 Can be calculated during the experiment. Review Experiment 13 for calculation. Atom Economy (%) Can be calculated during the experiment. Review Experiment 13 for calculation. Experimental Atom Economy (%) Cannot be calculated until the next lab period, as it is based on % yield! Review Experiment 13 for calculation. “Eproduct” Can be calculated after synthesis has been started based on the amount of each reagent used. Cost per bottle of reagent is given on p. 142. Cost per Synthesis ($) Cost per Gram ($/g) COST PER GRAM cannot be calculated without the value of actual product mass! PRODUCT ANALYSIS (Melting Point) The melting point of a pure solid is an important characteristic of the compound. A pure solid has a sharp characteristic melting point, but a solid that contains impurities will have a broader and lower melting point range. By comparing the experimental melting point to the literature value for pure p-methylacetanilide, the purity of the product can be determined. PRODUCT ANALYSIS (IR Spectroscopy) NH 2 1 2 6 5 3 4 CH 3 7 O H C N 8 CH 3 9 1 6 2 5 3 4 CH 3 7 Table 16.3 Functional Group Base Values p-methylaniline p-methylacetanilide Frequency (cm-1) Frequency (cm-1) Frequency (cm-1) Amines have two absorptions! Amides have one absorption! N-H stretch 3150-3500 sp2 CH stretch 3000-3100 sp3 CH stretch 2800-3000 C=O stretch 1650-1740 PRODUCT ANALYSIS (NMR Spectroscopy) 3H, s 2.2 d NH 2 1 H3,5 2H, d 6.9 d H2,6 2H, d 6.6 d 6 2 5 3 4 2H, s 3.5 d CH 3 7 O H 3H, s 2 .1 d C N 8 CH 3 9 3H, s 2 .3 d 1 6 2 5 3 H2,6 2H, d 7.4 d 4 CH 3 7 1H, s 7.9 d H3,5 2H, d 7.1 d Table 16.4 p-methylaniline p-methylacetanilide NH O NH 2 N H2, 6 1 H2, 6 2 5 3 H3,5 C2, 6 9 3 2 H7 5 3 C4 C7 4 3 CH H9 7 C2, 6 C3, 5 6 C3, 5 H7 CH 8 H3,5 4 CH C 1 C1 6 C1 H C7 NH 3 7 C8 C9 C4 Enter chemical shift ONLY in blank based on spectra on p. 138-139! SAFETY CONCERNS Nasty fumes are given off when the reaction mixture is heated. Acetic anhydride and acetic acid can cause burns. WASTE MANAGEMENT Slowly add 10% NaHCO3 to your combined filtrate (containing mainly water, acetic acid, and acetic anhydride) until vigorous bubbling of CO2 has ceased. Rinse down the drain in the hood. Next lab period, after you have weighed your amide product and determined its melting range, you will use it as the reactant for the next experiment! CLEANING All glassware should be cleaned with soap, water, and brush if necessary. Rinse all glassware with wash acetone before returning to lab drawer. DO NOT return any glassware to drawer wet or dirty!