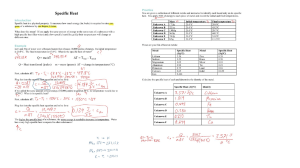
Unit 2: Energy & Rates of Reaction Thermochemistry is the study of heat change in chemical reactions. Chapter 5 5.1: Energy: is the capacity to do work (J) • Potential energy is the energy available by virtue of an object’s position • Kinetic energy is the energy of an object due to its motion • Thermal energy is the energy associated with the random motion of atoms and molecules • Chemical energy is the energy stored within the bonds of chemical substances • Nuclear energy is the energy stored within the collection of neutrons and protons in the atom 5.1 Energy Changes in Chemical Reactions Heat (q) is the transfer of thermal energy between two bodies that are at different temperatures. Temperature (T) is a measure of the average kinetic energy of the particles in a sample Thermal energy: the total quantity of kinetic and potential energy in a substance Temperature ≠ Thermal Energy 90℃ 40℃ greater thermal energy 5.1 Law of Conservation of Energy: Energy cannot be created or destroyed The system is the specific part of the universe that is of interest in the study. SYSTEM SURROUNDINGS open Exchange: mass & energy closed isolated energy nothing 5.1 Systems & Surroundings • Energy cannot be created or destroyed…but it can change forms and often it is ‘LOST’…as heat • The total energy of a system and its surroundings remains the same→LAW OF CONSERVATION OF ENERGY • Chemical System: the reactants and the products being studied • Surroundings: all matter not included within the system (reaction). Usually only consider anything nearby that is capable of exchanging energy or matter. Types of systems: • Open: both energy and matter can be transferred in and out of the system (ex: BBQ) • Closed: energy can enter and leave the system, but matter cannot (ex: glow stick) • Isolated: neither matter nor energy can move in or out of the system. • (Bond making is an) Exothermic process: is any process that gives off heat – transfers thermal energy from the system to the surroundings. • (bonds broken < bonds made). • It takes more energy to make bonds • System loses potential energy; products have a lower potential energy (and stronger bonds on average) than the reactants 2H2 (g) + O2 (g) H2O (g) 2H2O (l) + energy H2O (l) + energy 5.1 Endothermic process is any process in which heat has to be supplied to the system from the surroundings. • (bonds broken > bonds made) • takes more energy to break bonds • System gains potential energy; products have a higher potential energy (on average) and weaker bonds than the reactants energy + 2HgO (s) → 2Hg (l) + O2 (g) energy + H2O (s )→ H2O (l) Diagrams from p. 288-289 • • • • • Nuclear Energy All nuclear reactions are exothermic. Per unit of mass, nuclear reactions release much more energy than exothermic chemical reactions. Two main types of Reactions: Fusion and Fission A fusion reaction occurs when nuclei of small atomic mass combine to form larger molecule. 2 H + 3 H → 4 He + 1 n 1 1 2 0 In fission, large nuclei with high atomic mass are split to smaller, lighter mass by collision with a neutron: 235 92U + 1 0n → 92 36Kr + 141 1 n + 1.9 x 10 10 kJ/mol Ba + 3 56 0 See p. 290 Table 1 5.1 Nuclear Reactions For each of the following situations state whether the: • process is endothermic or exothermic • system or surroundings gain energy a) An electric current converts water into H2(g) and O2(g). Endothermic or exothermic system or surrounding gain b) Wood burning in a fireplace is converted into gases and leaves ashes. Endothermic or exothermic system or surrounding gain c) When ammonium chloride, a salt, is dissolved in water, the solution and beaker become cold. Endothermic or exothermic system or surrounding gain d) Iodine vapour changes directly to a solid. Endothermic or exothermic system or surrounding gain e) A hydrogen molecule which is composed of two atoms held together by electric forces is split into two separate atoms. Endothermic or exothermic system or surrounding gain f) Positively charged ions coalesce with negatively charged ions and form a solid ionic crystal. Endothermic or exothermic system or surrounding gain h) Solid carbon dioxide (dry ice) is converted directly into the gaseous phase. Endothermic or exothermic system or surrounding gain Enthalpy and Enthalpy Change What is enthalpy? Total amount of thermal energy (kinetic + potential energy) in a substance (kJ) • entHalpy… “Heat” of reaction Enthalpy Change (∆H): • Enthalpy cannot be measured, however energy changes in physical and chemical Reactions • The enthalpy change of the chemical system is equal to the flow of thermal energy in and out of the system, • ∆𝐻𝑠𝑢𝑟𝑟𝑜𝑢𝑛𝑑𝑖𝑛𝑔 > 0, Endothermic reaction • ∆𝐻𝑠𝑢𝑟𝑟𝑜𝑢𝑛𝑑𝑖𝑛𝑔 < 0, Exothermic reaction Energy Changes during Exothermic & Endothermic Reaction in an Open System DH = heat given off or absorbed during a reaction at constant pressure Hproducts < Hreactants DH < 0 Hproducts > Hreactants DH > 0 5.2 Molar Enthalphy Change Molar enthalpy, ∆𝐻𝑟𝑥𝑛 , the enthalpy change associated with a physical, chemical, or nuclear change involving one mole of a substance. Types of molar Enthalpies Dissolution (∆Hsol) NaBr(s) →Na+(aq) + Br-(aq) Combustion (∆Hcomb) CH4(g) + 2O2(g) → CO2(g) + H2O(l) Vapourization (∆Hvap) CH3OH(l) → CH3OH(g) Freezing (∆Hfr) H2O(l) → H2O(s) Neutralization (∆Hneut) 2NaOH(aq) + H2SO4(aq) →Na2SO4(aq) + H2O(l) Formation (∆Hf) C(s) +2H2(g) + 1/2O2(g) → CH3OH(l) ∆𝑯 𝒒 ∆𝑯𝒓𝒙𝒏 = ∆𝑯𝒓𝒙𝒏 = 𝒎𝒐𝒍 𝒎𝒐𝒍 Where n is the amount in mols and ∆Hr is the molar enthalpy change of the reaction 5.2 5.2 Molar Enthalpy Calculations Calculate ∆H for Vaporization Reactions Ethanol, CH3CH2OH(l), is used to disinfect the skin prior to an injection. If a 1.00 g sample of ethanol is spread across the skin and evaporated, what is the expected enthalpy change? The molar enthalpy of vaporization of ethanol is 38.6 kJ/mol. Given: methanol=1.00 g; ∆Hvap=38.6 kJ/mol MMethanol= 24 +6 +16 46g/mol Required = ∆H = q Analysis= q = n∆Hvap n= 1.00𝑔 46𝑔/𝑚𝑜𝑙 = 0.022 mol q = 0.022 mol x 38.6 kJ/mol = 0.849 kJ 5.2 Representing Molar Enthalpy Changes The equations we use to represent energy changes are called thermochemical equations. • Endothermic enthalpy changes are reported as positive values; H2O(l) → H2 + ½ O2(g) ∆Hdecomp = + 285.8 KJ/mol H2O • Exothermic enthalpy changes are reported as negative values H2(l) + ½ O2 (g) → H2O(l) ∆Hcomb = -285.8 KJ/mol H2 Energy changes can be communicated in four different ways. Three of them are thermochemical equations and one uses diagram. 1. By including an energy value as a term in the thermochemical equation e.g., CH3OH(l) + 3/2 O2 → CO2(g) + 2H2O(g) + 726 KJ (Exothermic Reaction) 2. By writing a chemical equation and stating its enthalpy change e.g., CH3OH(l) + 3/2O2(g)→CO2(g)+ 2H2O(g) ∆H = -726 KJ (Exothermic Reaction) 3. By stating the molar enthalpy of a specific reaction e.g., ∆Hcombustion or ∆Hc = - 726 KJ/mol CH3OH 5.2 4. Drawing a chemical potential energy diagram • Exothermic • Endothermic Potential Energy Diagram Is DH negative or positive? System absorbs heat Endothermic DH > 0 6.01 kJ are absorbed for every 1 mole of ice that melts at 00C and 1 atm. Thermochemical Equations H2O (s) H2O (l) DH = 6.01 kJ 5.2 Is DH negative or positive? System gives off heat Exothermic DH < 0 890.4 kJ are released for every 1 mole of methane that is combusted at 250C and 1 atm. Thermochemical Equations CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) DH = -890.4 kJ 5.2 Calculations in Thermochemical Equations • The stoichiometric coefficients always refer to the number of moles of a substance H2O (s) • DH = 6.01 kJ If you reverse a reaction, the sign of DH changes H2O (l) • H2O (l) H2O (s) DH = -6.01 kJ If you multiply both sides of the equation by a factor n, then DH must change by the same factor n. 2H2O (s) 2H2O (l) DH = 2 x 6.01 = 12.0 kJ 5.2 • The physical states of all reactants and products must be specified in thermochemical equations. H2O (s) H2O (l) H2O (l) DH = 6.01 kJ H2O (g) DH = 44.0 kJ How much heat is evolved when 266 g of white phosphorus (P4) burns in air? P4 (s) + 5O2 (g) 266 g P4 x P4O10 (s) 1 mol P4 123.9 g P4 x DHc = -3013 kJ/mol 3013 kJ = 6470 kJ 1 mol P4 5.2 Stoichiometry and Thermochemical Calculations • Enthalpy is dependent on the quantity of the products. Example: Aluminum reacts readily with chlorine gas to produce aluminum chloride. The enthalpy of this reaction is −1408 𝑘𝐽. What amount of heat is transferred when 1.0 kg of Al reacts completely with excess Cl2? 2Al(s) + 3Cl2(g) 2AlCl3 (s) + 1408 kJ ∆𝐻 𝑛 = −1408 𝑘𝐽 2 𝑚𝑜𝑙 𝐴𝑙 𝑛𝐴𝑙 = 1000 𝑔 = 37 𝑚𝑜𝑙 26.98 𝑔/𝑚𝑜𝑙 ∆𝐻 37 𝑚𝑜𝑙 = −1408 𝑘𝐽 2 𝑚𝑜𝑙 𝐴𝑙 ∆𝐻 = −26 048 𝑘𝐽 −1408 𝑘𝐽 refers to every 2 moles of Al(s) 𝑞 = ∆𝐻 = 𝑛∆𝐻𝑟 ∆𝐻𝑟 = ∆𝐻 𝑛 −1408 𝑘𝐽 𝑘𝐽 ∆𝐻𝑟 = = −704 2 𝑚𝑜𝑙 𝐴𝑙 𝑚𝑜𝑙 𝑞 = 𝑛𝐴𝑙 ∆𝐻𝑟 𝑘𝐽 𝑞 = 37 𝑚𝑜𝑙 × −704 𝑚𝑜𝑙 𝑞 = −26 048 𝑘𝐽 The amount of heat transferred is 26 048 𝑘𝐽 5.2 5.2: Calorimetry and Enthalpy The heat capacity (c) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. The specific heat (c) of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. Heat (q) absorbed or released: q = mcDt Dt = tfinal - tinitial 5.2 Calorimetry: the measurement of heat into or out of a system for chemical and physical processes. the heat released = the heat absorbed qsurroundings = - qsystem The device used to measure the absorption or release of heat in chemical or physical processes is called a “Calorimeter”; measure enthalpy changes for reactions. It works by insulating the system form the surroundings (ex. Styrofoam cup). 5.2 a “Calorimeter” Calorimetry Calculations When analyzing data obtained using a calorimeter, make these assumptions: • Any thermal energy transferred from the calorimeter to the outside environment is negligible. • Any thermal energy absorbed by the calorimeter itself is negligible. • All dilute, aqueous solutions have the same density (1.00 g/mL) and specific heat capacity (4.184 J/(g∙°C)) as water • thermal energy absorbed or released by a chemical system is given the symbol q . qwater = mcDt 5.2 Calculations Involving Thermal Energy Transfer Thermal Energy transfer by metal How much heat is given off when an 869 g iron bar cools from 940C to 50C? Solution: Given: c of Fe = 0.444 J/g • 0C m = 869 g Dt = tfinal – tinitial = 50C – 940C = -890C q = mcDt = 869 g x 0.444 J/g • 0C x (-890C) = -34,000 J No heat enters or leaves! = -34 kJ 5.2 Calculations of metal in water A student places 50.0 mL of liquid water at 21.00 °C into a coffee-cup calorimeter. She places a sample of gold at 100 °C into the calorimeter. The final temperature of the water is 21.33 °C. The specific heat capacity of water is 4.18 J/g∙°C and the density of water, d, is 1.00 g/mL. Calculate the quantity of thermal energy, q , absorbed by the water in the calorimeter. SOLUTION: Given: VH2O = 50.0 mL; c=4.18 J/(g∙°C); dH2O=1.00 g/mL; Tinitial=21.00 °C;Tfinal=21.33 °C Required: Quantity of thermal energy absorbed, q 𝑚 𝐷= 𝑣 m= vd, m = 50mL x 1.00g/mL = 50 g ∆t = 21.33oC – 21.00oC 5.2 q = mc∆t =50 g x 4.18 J x 0.33oC g. oC = 69 J Determining specific heat capacity of a substance Using the value of q above, calculate the specific heat capacity, c, of gold if its mass is 6.77 g. Final temperature of the gold is same as that of water in the calorimeter. Given:mAu =6.77 g; qsys = -69 J; t initial =100 oC; t final = 21.33oC ∆t =21.33 oC – 100oC = - 78.67 oC 𝑞 𝑐= Required: cAu 𝑚∆𝑇 −69𝐽 q = mc∆t 𝑐= Rearrange and solve for c (6.77 𝑔)(−78.67℃) 𝐽 𝑐 = 0.129 𝑔℃ Thermal Energy Transfer during a Neutralization reaction A 50.0 mL sample of a 1.0 mol/L aqueous solution of hydrochloric acid, HCl(aq), was mixed with 50.0 mL of a 1.0 mol/L aqueous solution of sodium hydroxide, NaOH(aq), at 25.0 °C in a calorimeter. After the solutions were mixed by stirring, the temperature was 31.9 °C. (a) Determine the quantity of thermal energy transferred by the reaction to the water (surroundings), q , Assume that the specific heat capacity and density of both solutions is the same as that of liquid water (b) State whether the reaction was endothermic or exothermic. Given: Total volume = 100 mL; c water= 4.18J/g. oC dwater = m =1g/mL v m = vd; msolution = 100 g ∆t = 31.9 oC – 25.0 oC = 6.9 oC; q surr= mc∆t =100 g x 4.18 J x 6.9oC g• oC = 2.9 x103 J = + 2.9 kJ q surr= -qsystem +2.9 kJ = -2.9 kJ Exo.