Thermodynamics Problem Set: Heat Transfer & Processes
advertisement

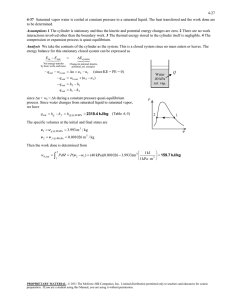
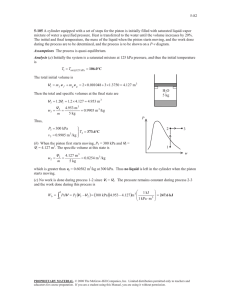
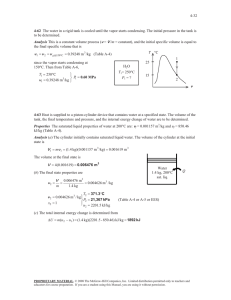
1. Steam enters the condenser of a steam power plant at 20 kPa and a quality of 95 percent with a mass flow of 20000 kg/h. It is to be cooled by water from a nearby river by circulating the water through the tubes within the condenser. To prevent thermal pollution, the river water is not allowed to experience a temperature rise above 10oC. If the steam is to leave the condenser as saturated liquid at 20 kPa, determine the mass flow rate of the cooling water required. 3 2 1 4 Solution: Steam is condensed by cooling water in the condenser of a power plant. If the temperature rise of the cooling water does not exceed 10oC, determine the minimum mass flow rate of the cooling water. Assumptions: 1). This is a steady flow process since there is no change in properties with time. 2). Kinetic and potential energy changes are negligible. 3). There are no work interactions. 4). Heat loss from the device to the surroundings is negligible and thus heat transfer from the hot fluid is equal to the heat transfer to the cold fluid. 5). Liquid water is an incompressible substance with constant specific heats at room temperature. Properties: The cooling water exists as compressed liquid at both states, and its specific heat at room temperature is c = 4.18 kJ/kgK (Table A 4). The enthalpies of the steam at the inlet and the exit states are: 𝑃3 = 20 𝑘𝑃𝑎 𝑥3 = 0.95 therefore ℎ3 = ℎ𝑓 + 𝑥ℎ𝑓𝑔 = 251.38 + 0.95(2358.33) = 2491.8 𝑘𝐽/𝑘𝑔 𝑃4 = 20 𝑘𝑃𝑎 saturated liquid from here ℎ4 = ℎ𝑓@20 𝑘𝑃𝑎 = 251.38 𝑘𝐽/𝑘𝑔 We take the heat exchanger as the system, which is a control volume. The mass and energy balance for this steady flow system can be expressed in the rate form as: Mass balance (for each fluid steam): 𝑚̇𝑖 − 𝑚̇𝑒 = ∆𝑚̇𝐶𝑉 steady state, so 𝑚̇𝑖 = 𝑚̇𝑒 𝑚̇1 = 𝑚̇2 = 𝑚̇𝑤𝑎𝑡𝑒𝑟 𝑚̇3 = 𝑚̇4 = 𝑚̇𝑠𝑡𝑒𝑎𝑚 Energy balance under stated assumptions becomes: 𝑚̇1 ℎ1 + 𝑚̇3 ℎ3 = 𝑚̇2 ℎ2 + 𝑚̇4 ℎ4 Combining the two: 𝑚̇𝑤𝑎𝑡𝑒𝑟 (ℎ1 − ℎ2 ) = 𝑚̇𝑠𝑡𝑒𝑎𝑚 (ℎ3 − ℎ4 ) Solving for 𝑚̇𝑤𝑎𝑡𝑒𝑟 : 𝑚̇𝑤𝑎𝑡𝑒𝑟 = = ℎ3 − ℎ4 ℎ3 − ℎ4 𝑚̇𝑠𝑡𝑒𝑎𝑚 = 𝑚̇ ℎ2 − ℎ1 𝐶𝑝 (𝑇2 − 𝑇1 ) 𝑠𝑡𝑒𝑎𝑚 (2491.7 − 251.38) (20000/3600 𝑘𝑔/𝑠) = 297 𝑘𝑔/𝑠 𝑘𝐽 (10℃) (4.18 ) 𝑘𝑔℃ 2. In a steady state process, the high-pressure water at 20 MPa and 300°C with flow rate 1 m3/min is throttled into an 1000 lit/min evaporator chamber which forms liquid and vapor at a lower pressure as shown in the figure. Saturated vapor having quality x =1 comes out of outlet A, while saturated water (x=0) having specific volume 0.00103 m3/kg comes out of the outlet B. Calculate: a). the mass flow rate (kg/s) of incoming stream. mediate equilibrium (state 2). Subsequently, the nly. Estimate the net heat transferred to air while to state 3 via state 2. ved simultaneously and suddenly, estimate heat g the system directly from state 1 to state 3. be 10 m/s2] (12 Points) b). Estimate, temperature, pressure, and mass flow rate (kg/s) of saturated water stream me of superheated water vapor at 10 MPa and of outlet B. steam tables (2 Points), quation (2 Points), (ii) the ssibility chart (4 Points). ss, the high Compr essed water d 300°C with 20 MPa and 300°C 1000 lit/min tled into an ms liquid and Outl et A shown in the satur ated steam, x=1 quality x =1 ile saturated Evaporator ecific volume Outl et B the outlet B. satur ated water te (kg/s) of v=1.03×10–3m3/kg Estimate, ass flow rate (kg/s) of saturated water stream of Solution: Outlet B (saturated water x = 0): Page 1 of 2 𝑣𝐵 = 0.00103 𝑚3 (𝑔𝑖𝑣𝑒𝑛) 𝑘𝑔 look for this volume at table TB1.1 and interpolate to find TB and PB. TB = 81.6oC and PB = 50.87 kPa To find hB therefore you look at TB1.2 at PB = 50.87 kPa and T ≈ 81.33℃ ℎ𝐵 = 340.47 𝑘𝐽/𝑘𝑔 Outlet A (saturated vapor x = 1): Use T B1.2 at 50 kPa: ℎ𝑔𝐴 = 2645.87 𝑘𝐽/𝑘𝑔 𝑣𝑔𝐴 = 3.24034 𝑚3 /𝑘𝑔 Mass balance equation: 𝑚̇1 = 𝑚̇𝐴 + 𝑚̇𝐵 𝑚̇𝐴 + 𝑚̇𝐵 = 12.5 𝑘𝑔/𝑠 𝑚̇1 = 𝑉̇ 0.017𝑚3 /𝑠 = = 12.5 𝑘𝑔/𝑠 𝑣1 0.00136 𝑚3 /𝑘𝑔 0.00136 𝑚3 𝑣1 = (𝑇𝑎𝑏𝑙𝑒 𝐵. 1.4 𝑎𝑡 20000 𝑘𝑃𝑎 𝑎𝑛𝑑 300℃) 𝑘𝑔 Energy balance: 𝑚̇1 ℎ1 = 𝑚̇𝐴 ℎ𝐴 + 𝑚̇𝐵 ℎ𝐵 12.5 𝑥 1333.29 = 𝑚̇𝐴 𝑥2645.87 + 𝑚̇𝐵 𝑥 340.47 12.5𝑥 1333.29 = 2645.87(12.5 − 𝑚̇𝐵 ) + 𝑚̇𝐵 𝑥 340.47 𝑚̇𝐵 = 7.1168 𝑘𝑔/𝑠 3. A well-insulated piston–cylinder assembly is connected by a valve to an air supply line at 8 bar (1 bar=100 kPa), as shown in the figure. Initially (state 1), the air inside the cylinder is at 1 bar, 300 K, and the piston is located 0.5 m above the bottom of the cylinder. The atmospheric pressure is 1 bar, and the diameter of the piston face is 0.3 m. The valve is opened and air is admitted slowly until the volume of air inside the cylinder has doubled (state 2). The weight of the piston and the friction between the piston and the cylinder wall can be ignored. Using the ideal gas model, obtain a relationship between final temperature (T2) and temperature of air supply line (Ts). Estimate the final temperature, in K, and mass, in kg, of the air inside the cylinder for supply temperatures: of Ts =500 K. ents of the second law of thermodynamics by Kelvin – 4 Points) ed piston–cylinder by a valve to an air (1 bar=100 kPa), as re. Initially (state 1), er is at 1 bar, 300 K, ted 0.5 m above the r. The atmospheric the diameter of the e valve is opened and ntil the volume of air doubled (state 2). The d the friction between ylinder wall can be l gas model, obtain a nal temperature (T2 ) supply line (Ts). Estimate the final temperature, in K, and nside the cylinder for supply temperatures: of Ts =500 K. Solution: static (quasiequilibrium) process, and (II) A reversible Supply line state: compressor is to be upled adiabatic steam ving a generator. Steam 2.5 MPa and 500°C at a xits at 10 kPa and a rs the compressor at 98 of 10 kg/s and exits at termine the net power enerator by the turbine. (12 Points) is rotated in a rigid and insulated closed vessel with 𝑃𝑙 = 800 𝑘𝑃𝑎 Cylinder: Initially (state 1): 𝑃1 = 100 𝑘𝑃𝑎; 𝑇1 = 300 𝐾 Final state (state 2): 𝑃2 = 100 𝑘𝑃𝑎; 𝑇2 =? Mass balance equation: 𝑚𝑖 = 𝑚2 − 𝑚1 Energy equation: 𝑚2 𝑢2 − 𝑚1 𝑢1 = −𝑊1−2 + (𝑚2 − 𝑚1 )ℎ𝑙 ℎ𝑙 = ℎ𝑖 ∆𝑉 = 2𝑉1 − 𝑉1 = 𝑉1 = 𝜋 (0.15)2 𝑥0.5 = 0.03534 𝑚3 𝑉1 = 𝐴ℎ1 = 0.03534 𝑚3 𝑉2 = 2𝐴ℎ1 = 0.07068 𝑚3 Work term: 𝑊 = 𝑃∆𝑉 = 100 𝑘𝑃𝑎 (0.03534 𝑚3 ) = 3.534 𝑘𝐽 Enthalpy: ℎ𝑙 = 𝑐𝑝 𝑇𝑙 Cp air = 1.004 kJ/kg.K Initial mass: 𝑚1 = 𝑃1 𝑉1 100𝑥 0.03534 𝑚3 = = 0.04105 𝑘𝑔 𝑅𝑇1 0.287 𝑥 300 𝑚2 = 𝑃2 𝑉2 100𝑥 0.07068 𝑚3 24.627 = = 𝑅𝑇2 0.287 𝑥 𝑇2 𝑇2 Internal energy term: 𝑢2 = 𝑐𝑣 𝑇2 = 0.717𝑇2 Substitute in the main energy equation: 24.627 24.627 𝑥 0.717𝑇2 − 215.4 𝑥 0.04105 = ( − 0.04105 ) 𝑥1.004𝑇𝑙 − 3.534 𝑇2 𝑇2 for Tline = 500K 12.312 24.627 = − 0.04105 𝑇𝑙 𝑇2 𝑇2 = 375𝐾 𝑚2 = 0.065𝑘𝑔 4. A tank having a volume of 0.85 m3 initially contains water as a two-phase liquid-vapor mixture at 260oC and a quality of 0.7. Saturated water vapor at 260oC is slowly withdrawn through a pressure-regulating valve at the top of the tank as energy is transferred by heat to maintain the pressure constant in the tank. This continues until the tank is filled with saturated vapor at 260oC. Determine the amount of heat transfer, in kJ. Solution: 1. For the CV, 𝑊𝐶𝑉 = 0 and kinetic and potential energy effects can be neglected. 2. At the exit, the state remains constant. 3. The initial and final states of the mass within the vessel are equilibrium states. Since there is a singe exit and no inlet, the mass rate balance takes form: 𝑑𝑚𝐶𝑉 = −𝑚𝑒 𝑑𝑡 With assumption 1, the energy rate balance: 𝑑𝐸𝐶𝑉 𝑉12 𝑉12 = 𝑄𝐶𝑉 − 𝑊𝐶𝑉 + ∑ 𝑚𝑖 (ℎ𝑖 + + 𝑔𝑧𝑖 ) − ∑ 𝑚𝑒 (ℎ𝑒 + + 𝑔𝑧𝑒 ) 𝑑𝑡 2 2 𝑖 reduces to: 𝑒 𝑑𝑈𝐶𝑉 = 𝑄𝐶𝑉 − 𝑚𝑒 ℎ𝑒 𝑑𝑡 Combining the mass and energy rate balances results in: 𝑑𝑈𝐶𝑉 𝑑𝑚𝐶𝑉 = 𝑄𝐶𝑉 + ℎ𝑒 𝑑𝑡 𝑑𝑡 By assumption 2, the specific enthalpy at the exit is constant. Accordingly, integration of the last equation gives: ∆𝑈𝐶𝑉 = 𝑄𝐶𝑉 + ℎ𝑒 ∆𝑚𝐶𝑉 Solving for the heat transfer 𝑄𝐶𝑉 : 𝑄𝐶𝑉 =∆𝑈𝐶𝑉 − ℎ𝑒 ∆𝑚𝐶𝑉 𝑄𝐶𝑉 = (𝑚2 𝑢2 − 𝑚1 𝑢1 ) − ℎ𝑒 (𝑚2 − 𝑚1 ) where m1 and m2 denote, respectively, the initial and final amounts of mass within the tank. The terms u1 and m1 of the above equation can be evaluated with property values from Table at 260oC and the given value for quality. Thus: 𝑢1 = 𝑢𝑓 + 𝑥1 (𝑢𝑔 − 𝑢𝑓 ) = 1128.4 + (0.7)(2599.0 − 1128.4) = 2157.8 𝑘𝐿/𝑘𝑔 Table B1.1 𝑣1 = 𝑣𝑓 + 𝑥1 (𝑣𝑔 − 𝑣𝑓 ) = 1.2755 𝑥 10−3 + 0.7(0.04221 − 1.2755𝑥10−3 ) = 29.93𝑥10−3 𝑚3 /𝑘𝑔 Using the specific volume v1 the mass initially contained in the tank is: 𝑚1 = 𝑉 0.85 𝑚3 = = 28.4 𝑘𝑔 𝑣1 29.93𝑥10−3 𝑚3 /𝑘𝑔 The final state of the mass in the tank is saturated vapor at 260C so from the table: 𝑢2 = 𝑢𝑔 (260℃) = 2599.0 𝑘𝐽/𝑘𝑔 𝑣2 = 𝑣𝑔 (260℃) = 42.21𝑥10−3 𝑚3 /𝑘𝑔, Table B1.1 The mass contained within the tank at the end of the process is: 𝑉 0.85 𝑚3 𝑚2 = = = 20.14 𝑘𝑔 𝑣2 42.21𝑥10−3 𝑚3 /𝑘𝑔 Table also gives: ℎ𝑒 = ℎ𝑔 (260℃) = 2796.89 𝑘𝐽/𝑘𝑔 Substituting values into the expression for the heat transfer yields: 𝑄𝐶𝑉 = (𝑚2 𝑢2 − 𝑚1 𝑢1 ) − ℎ𝑒 (𝑚2 − 𝑚1 ) 𝑄𝐶𝑉 = 20.14𝑥2599.0 − 28.4𝑥2157.8 − 2796.6(20.14 − 28.4) = 14162 𝑘𝐽 In this case, idealizations are made about the state of the vapor exiting and the initial and final states of the mass contained within the tank. 5. A vessel has a volume of 1.45 m3. Initially, 80% of its volume is filled with saturated liquid ammonia at -25oC and the rest is filled with ammonia vapor. Liquid leaves through a small valve while heat is added to maintain the temperature at -25oC. The final mass of liquid in the vessel is one-third of the initial mass of liquid. Determine the amount of heat that must be added. 𝑁𝐻3 , 𝑡𝑟𝑎𝑛𝑠𝑖𝑒𝑛𝑡 𝑝𝑟𝑜𝑏𝑙𝑒𝑚 (𝑓𝑖𝑟𝑠𝑡 𝑙𝑎𝑤 𝑎𝑛𝑎𝑙𝑦𝑠𝑒𝑠) Both states (1 and 2) are saturated at T = -25oC. NH3 Q State 1: 𝑚𝑔1 = 𝑚𝑓1 = 0.2𝑉 0.2(1.45) = = 0.376 𝑘𝑔 𝑣𝑔 0.7712 0.8𝑉 0.8(1.45) = = 778.52 𝑘𝑔 𝑣𝑔 0.001490 𝑚1 = 𝑚𝑓1 + 𝑚𝑔1 State 2: 𝑚𝑓2 = 𝑚𝑓1 778.52 = = 259.5 𝑘𝑔 3 3 𝑉𝑓2 = 𝑚𝑓2 𝑣𝑓 = (259.5)(0.001490) = 0.387 𝑚3 𝑉𝑔2 = 𝑉 − 𝑉𝑓2 = 1.45 − 0.387 = 1.063𝑚3 𝑚𝑔2 = 𝑉𝑔2 1.063 = = 1.379 𝑘𝑔 𝑣𝑔 0.7712 𝑚2 = 𝑚𝑓2 + 𝑚𝑔2 𝑚𝑔1 𝑢𝑔 + 𝑚𝑓1 𝑢𝑓 + 1Q2 = 𝑚𝑔2 𝑢𝑔 + 𝑚𝑓2 𝑢𝑓 − (𝑚1 − 𝑚2 )ℎ𝑓 1Q2 = (𝑚𝑔2 − 𝑚𝑔1 )𝑢𝑔 − (𝑚𝑓2 − 𝑚𝑓1 )𝑢𝑓 + (𝑚1 − 𝑚2 )ℎ𝑓 = 1350 kJ 6. Acetylene initially at 95 kPa, 40oC is held in an insulated tank having a volume of 0.30m3. Heat is added to the system at a constant rate of 100W. Acetylene is bled from the tank to hold the pressure constant. Assuming, that conditions are uniform, determined the time required for the acetylene to reach a temperature of 120 oC. Solution: Assumptions: 1). Assume an ideal gas 2). Neglect KE and PE effects 3). 𝑊1−2 = 0 𝑚2 𝑢2 − 𝑚1 𝑢1 = 𝑄1−2 − 𝑊1−2 + 𝑚𝑖 ℎ𝑙 − 𝑚𝑒 ℎ𝑒 (𝑚2 − 𝑚1 ) = 𝑚𝑒 = 0.058 𝑘𝑔 Table A5: 𝐶𝑣 = 1.38 𝑘𝐽/𝑘𝑔𝐾 𝐶𝑝 = 1.699 𝑘𝐽/𝑘𝑔𝐾 𝑢2 = 𝐶𝑣 𝑇2 = (1.38)(120℃ + 273.15) = 542.55 𝑘𝐽/𝑘𝑔 𝑢1 = 𝐶𝑣 𝑇1 = (1.38)(40℃ + 273.15) = 432.15 𝑘𝐽/𝑘𝑔 𝑚1 = 𝑃1 𝑉1 (95𝑘𝑃𝑎)(0.3) = = 0.285 𝑘𝑔 𝑅𝑇1 (0.3193)(313.15) 𝑚2 = 𝑃2 𝑉2 (95𝑘𝑃𝑎)(0.3) = = 0.227 𝑘𝑔 𝑅𝑇2 (0.3193)(393.15) (𝑚2 − 𝑚1 ) = 𝑚𝑒 = 0.058 𝑘𝑔 ℎ𝑒 = ℎ1 + ℎ2 2 ℎ1 = 𝐶𝑝 𝑇1 = (1.699)(313.15) = 532.04 𝑘𝐽/𝑘𝑔 ℎ2 = 𝐶𝑝 𝑇2 = (1.699)(393.15) = 667.96 𝑘𝐽/𝑘𝑔 𝑘𝐽 ℎ𝑒 = 600 𝑘𝑔 𝑄1−2 = 𝑚2 𝑢2 − 𝑚1 𝑢1 + 𝑚𝑒 ℎ𝑒 = = (0.227)(542.55) − (0.285)(432.15) + (0.058)(600) = 34.796 𝑘𝐽 ∆𝑡 = 𝑄1−2 (34.796)𝑥103 𝐽 = = 347.96 𝑠𝑒𝑐 100 𝐽/𝑠 𝑄̇ 7. A rigid container has two rooms filled with water, each 1 m3 separated by a wall (see fig). Room A has P = 200 kPa with a quality x = 0.80. Room B has P = 2 MPa and T = 400°C. The partition wall is removed and the water comes to a uniform state, which after a while due to heat transfer has a temperature of 200°C. Find the final pressure and the heat transfer in the process. 8. A water cooler for drinking water should cool 25 L/h water from 18oC to 10oC using a small refrigeration unit with a COP of 2.5. Find the rate of cooling required and the power input to the unit. 9. A cyclic machine, receives 325 kJ from a 1000 K energy reservoir. It rejects 125 kJ to a 400 K energy reservoir and the cycle produces 200 kJ of work as output. Is this cycle reversible, irreversible, or impossible? 10. A household freezer operates in a room at 20°C. Heat must be transferred from the cold space at a rate of 2 kW to maintain its temperature at −30°C. What is the theoretically smallest (power) motor required to operate this freezer? 11. As shown below, a Carnot refrigeration cycle is performed in a closed system in the region of saturated liquid–vapor mixture with 0.6 kg of R-134a. The maximum and minimum temperatures of the cycle are shown in the figure. At the end of the heat rejection process, the refrigerant is saturated liquid and the input network to the cycle is 12 kJ. Determine: a) the fraction of the mass of the refrigerant that vaporizes during the heat addition process b) the pressure at the end of the heat rejection process s Knowing the high and low temperatures, the coefficient of performance of the cycle is: 𝐶𝑂𝑃𝑅 = 1 1 = = 9.464 𝑇𝐻 /𝑇𝐿 − 1 (20 + 273𝐾)/(−8 + 273 𝐾) − 1 The amount of cooling is determined from the definition of the coefficient of performance to be: 𝑄𝐿 = 𝐶𝑂𝑃𝑅 × 𝑊𝑖𝑛 = 9.464 × 12 = 113.5 𝑘𝐽 The enthalpy of vaporization R-134a at -8℃ is ℎ𝑓𝑔 = 204.59 kJ/kg (Table B.5.1). Then the amount of refrigerant that vaporizes during heat absorption becomes: 𝑄𝐿 = 𝑚𝑒𝑣𝑎𝑝 ℎ𝑓𝑔@−8℃ → 𝑚𝑒𝑣𝑎𝑝 = 113.5 𝑘𝐽 = 0.554 𝑘𝑔 204.59 kJ/kg Therefore, the fraction of mass that vaporized during heat addition process to the refrigerant is: 𝑚𝑒𝑣𝑎𝑝 0.554 𝑀𝑎𝑠𝑠 𝑓𝑟𝑎𝑐𝑡𝑖𝑜𝑛 = = = 0.93 𝑜𝑟 93% 𝑚𝑡𝑜𝑡𝑎𝑙 0.6 The pressure at the end of heat rejection process is simply the saturation pressure at heat rejection temperature: 𝑃4 = 𝑃𝑠𝑎𝑡@20℃ = 572.8 𝑘𝑃𝑎 12. A Carnot heat engine gets heat from a reservoir at 900 ℃ and with rate of 800 kJ/min. It rejects the waste heat to the ambient air at 27 ℃. The whole work output of the heat engine is used for a refrigerator that removes heat from the refrigerated space at -5℃ and transfer that to the same ambient air at 27℃. Determine: a) the maximum rate of heat removal from the refrigerated space b) the total rate of heat rejection to the ambient air a) The highest thermal efficiency a heat engine operating between two specified temperature limits can have is the Carnot efficiency, which is determined from: Ƞ𝑡ℎ,𝑚𝑎𝑥 = Ƞ𝑡ℎ,𝐶 = 1 − 𝑇𝐿 300 𝐾 =1− = 0.744 𝑇𝐻 1173 𝐾 Then the maximum power output of this heat engine is determined from the definition of thermal efficiency to be: 𝑊̇𝑛𝑒𝑡,𝑜𝑢𝑡 = Ƞ𝑡ℎ 𝑄̇𝐻 = 0.744 ∗ 800 = 595.2 𝑘𝐽/𝑚𝑖𝑛 which is also the power input to the refrigerator: The rate of heat removal from the refrigerated space will be a maximum if a Carnot refrigerator is used. The COP of the Carnot refrigerator is: 𝐶𝑂𝑃𝑅,𝑟𝑒𝑣 = 1 1 = = 8.37 𝑇𝐻 /𝑇𝐿 − 1 (27 + 273)/(−5 + 273) − 1 Then the rate of heat removal from the refrigerated space becomes: 𝑄̇𝐿,𝑅 = 𝐶𝑂𝑃𝑅,𝑟𝑒𝑣 (𝑊̇𝑛𝑒𝑡,𝑖𝑛 ) = 8.37 ∗ 595.2 = 4982 𝑘𝐽/𝑚𝑖𝑛 b) 𝑄̇𝐿,𝐻𝐸 = 𝑄̇𝐻,𝐻𝐸 − 𝑊̇𝑛𝑒𝑡,𝑜𝑢𝑡 = 800 − 595.2 = 204.8 𝑘𝐽/𝑚𝑖𝑛 𝑄̇𝐻,𝑅 = 𝑄̇𝐿,𝑅 + 𝑊̇𝑛𝑒𝑡,𝑖𝑛 = 4982 + 595.2 = 5577.2 𝑘𝐽/𝑚𝑖𝑛 And 𝑄̇𝑎𝑚𝑏𝑖𝑒𝑛𝑡 = 𝑄̇𝐿,𝐻𝐸 + 𝑄̇𝐻,𝑅 = 204.8 + 5577.2 = 5782 𝑘𝐽/𝑚𝑖𝑛 13. A room which is 4m*5m*8m is being heated using an electric resistance heater that is located in a short duct inside the room. At first, the room temperature is 15℃ and the local atmosphere pressure is 98 kPa. The room steadily loses heat to the outside with the rate of 150 kJ/min. There is a 200W fan that circulates the air through the duct and the heater at the average mass flow rate of 40 kg/min. The duct is adiabatic. There is no air leakage in or out the room. To reach the average temperature of 25℃ of the room air, it takes 20 minutes. Determine: the power rating of the electric heater The total mass of air in the room is: 𝑉 = 4 ∗ 5 ∗ 8 = 160 𝑚3 𝑚= 𝑃1 𝑉 98 𝑘𝑃𝑎 ∗ 160 𝑚3 = = 189.7 𝑘𝑔 𝑅𝑇1 (0.287 𝑘𝑃𝑎. 𝑚3 /𝑘𝑔. 𝐾)(288 𝐾) We first take the entire room as our system, which is a closed system since no mass leaks in or out. The power rating of the electric heater is determined by applying the conservation of energy relation to this constant volume closed system: 𝐸𝑖𝑛 − 𝐸𝑜𝑢𝑡 = ∆𝐸𝑠𝑦𝑠𝑡𝑒𝑚 𝑊𝑒,𝑖𝑛 + 𝑊𝑓𝑎𝑛,𝑖𝑛 − 𝑄𝑜𝑢𝑡 = ∆𝑈 ∆𝑡(𝑊̇𝑒,𝑖𝑛 + 𝑊̇𝑓𝑎𝑛,𝑖𝑛 − 𝑄̇𝑜𝑢𝑡 ) = 𝑚𝑐𝑣,𝑎𝑣𝑔 (𝑇2 − 𝑇1 ) Solving for the electrical work input gives: 𝑊̇𝑒,𝑖𝑛 = 𝑄̇𝑜𝑢𝑡 − 𝑊̇𝑓𝑎𝑛,𝑖𝑛 + 𝑚𝑐𝑣,𝑎𝑣𝑔 (𝑇2 − 𝑇1 )/∆𝑡 = 150 𝑘𝐽 𝑘𝐽 1 ( 𝑘𝐽/𝑠) − (0.2 ) + (189.7 𝑘𝑔) (0.718 . ℃) (25 − 15)℃ = 3.435 𝑘𝑊 60 𝑠 𝑘𝑔 20 ∗ 60 14. A piston/cylinder contains 50 kg of water at 200 kPa with a volume of 0.1 m3 . Stops in the cylinder are placed to restrict the enclosed volume to a maximum of 0.5 m3 . The water is now heated until the piston reaches the stops. Find the necessary heat transfer. 15. A piston/cylinder contains air at 1380 K, 15 MPa, with V1 = 10 cm3, Acyl= 5 cm2. The piston is released, and just before the piston exits the end of the cylinder the pressure inside is 200 kPa. If the cylinder is insulated, what is its length? How much work is done by the air inside? 16. A closed tank, V = 10 L, containing 5 kg of water initially at 25°C, is heated to 175°C by a heat pump that is receiving heat from the surroundings at 25°C. Assume that this process is reversible. Find the heat transfer to the water and the change in entropy. 17. A mass of 1 kg of air contained in a cylinder at 1.5 MPa, 1000 K, expands in a reversible isothermal process to a volume 10 times larger. Calculate the heat transfer during the process and the change of entropy of the air. 18. A spring loaded piston cylinder contains 1.5 kg air at 27oC and 160 kPa. It is now heated in a process where pressure is linear in volume, P = A + BV, to twice the initial volume where it reaches 900 K. Find the work, the heat transfer and the total entropy generation assuming a source at 900 K.