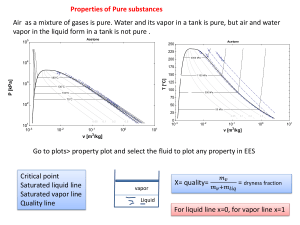
4-27 to be determined. 3
advertisement
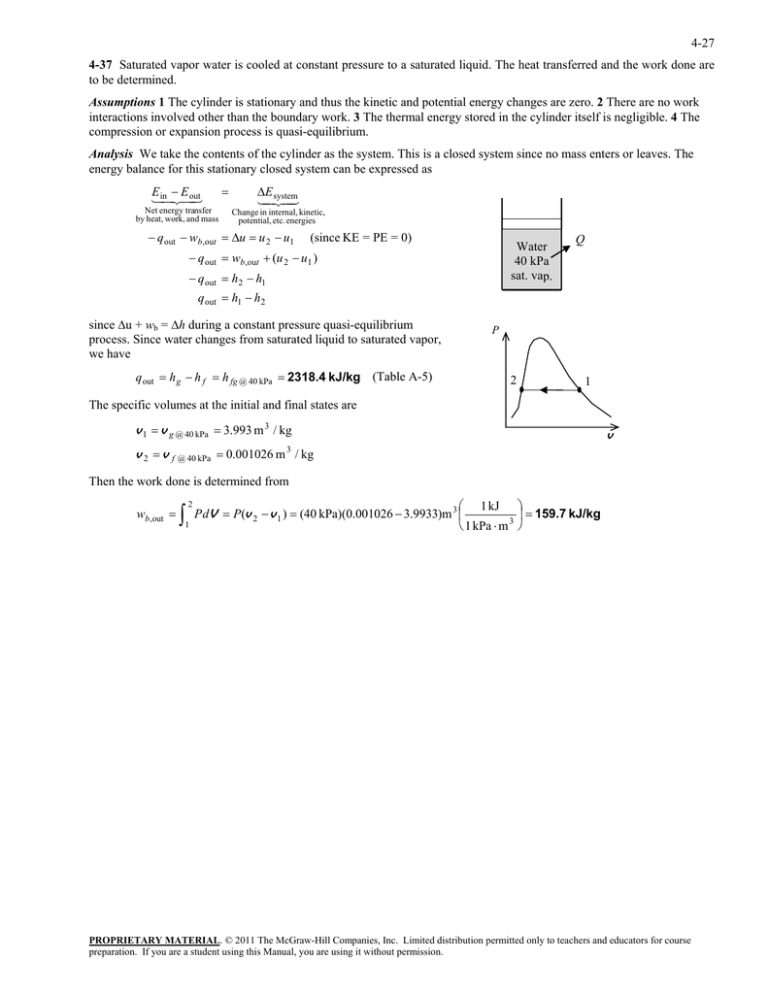
4-27 4-37 Saturated vapor water is cooled at constant pressure to a saturated liquid. The heat transferred and the work done are to be determined. Assumptions 1 The cylinder is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions involved other than the boundary work. 3 The thermal energy stored in the cylinder itself is negligible. 4 The compression or expansion process is quasi-equilibrium. Analysis We take the contents of the cylinder as the system. This is a closed system since no mass enters or leaves. The energy balance for this stationary closed system can be expressed as E E inout Net energy transfer by heat, work, and mass E system Change in internal, kinetic, potential, etc. energies q out wb,out u u 2 u1 (since KE = PE = 0) Water 40 kPa sat. vap. q out wb,out (u 2 u1 ) q out h2 h1 Q q out h1 h2 since u + wb = h during a constant pressure quasi-equilibrium process. Since water changes from saturated liquid to saturated vapor, we have q out h g h f h fg @ 40 kPa 2318.4 kJ/kg (Table A-5) P 2 1 The specific volumes at the initial and final states are v 1 v g @ 40 kPa 3.993 m 3 / kg v v 2 v f @ 40 kPa 0.001026 m / kg 3 Then the work done is determined from wb,out 2 1 1 kJ P dV P(v 2 v 1 ) (40 kPa)(0.001026 3.9933)m 3 159.7 kJ/kg 1 kPa m 3 PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.